E. Update Testing Site
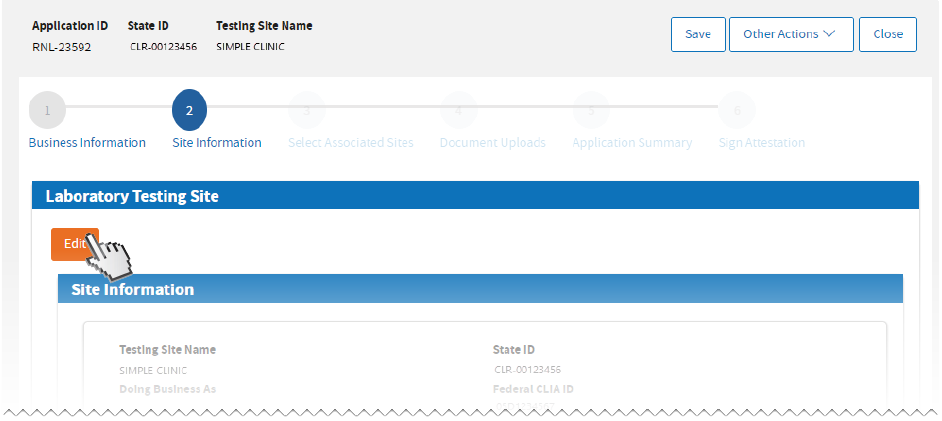
 Click
Edit to update
Testing Site Information to update
Tests Performed, Testing Personnel, and Physical Address.
Click
Edit to update
Testing Site Information to update
Tests Performed, Testing Personnel, and Physical Address.
 Complete all the required fields. Click
Edit, verify or update the address, then click
Validate first before clicking
Next.
Complete all the required fields. Click
Edit, verify or update the address, then click
Validate first before clicking
Next.
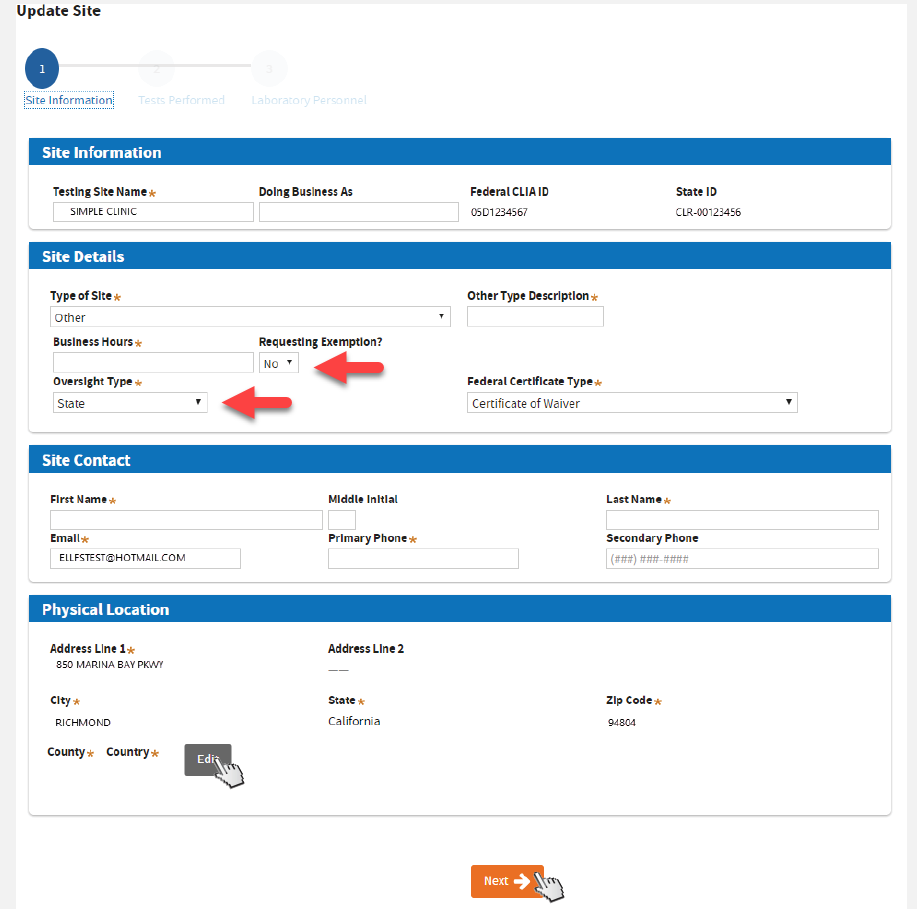 |
If
Requesting Exemption under BPC 1241, select
Yes. If you choose Yes but you don’t qualify, your application might be delayed.
|
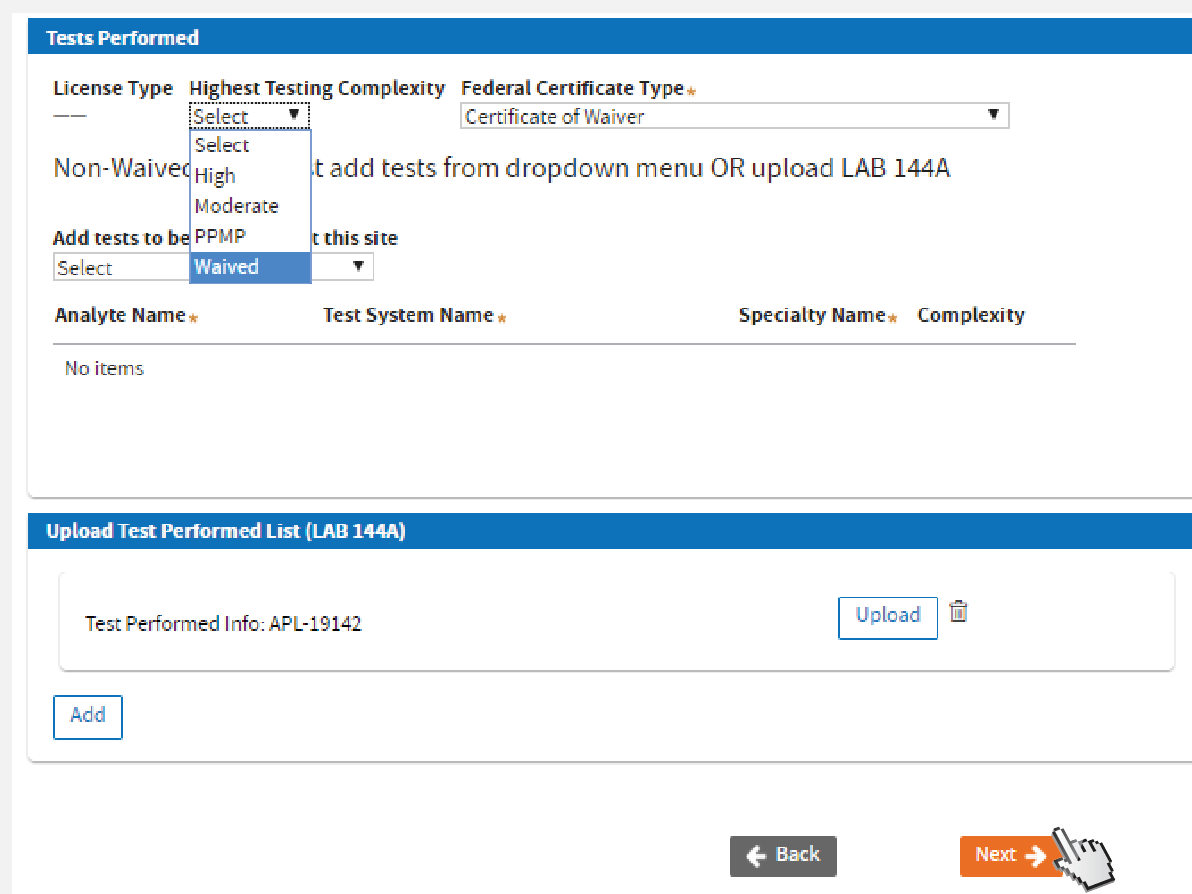
 Update Tests Performed:
Update Tests Performed:
Selecting the appropriate
Highest Testing Complexity and
Federal Certificate Type, will help the system to identify your License Type.
 |
Refer to the
table below as guide to select the matching options to avoid error and delay.
|
|
Highest Testing Complexity |
Federal Certificate Type |
|
If selecting... |
select... |
| High |
Certificate of Compliance or Certificate of Accreditation |
| Moderate |
Certificate of Compliance or Certificate of Accreditation |
| PPMP |
Certificate of Provider Performed Microscopy Procedures |
| Waived |
Certificate of Waiver |
Uploading LAB 144A is not required if Certificate Type is Waived or PPMP. Click
Next.
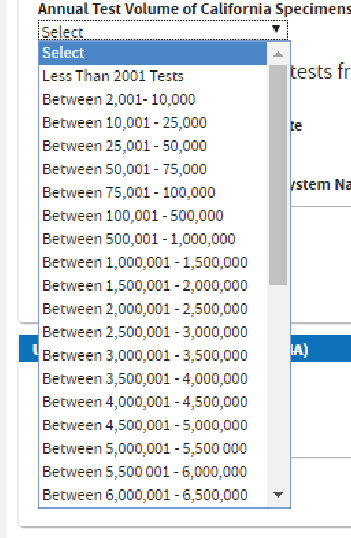
Based on the current test volume, select the
Annual Test Volume of California Specimens.
Enter the
Analyte, Test System, Specialty, and Complexity one by one...
Select The Federal Database to add tests.
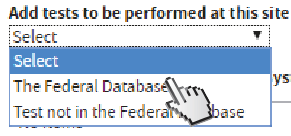
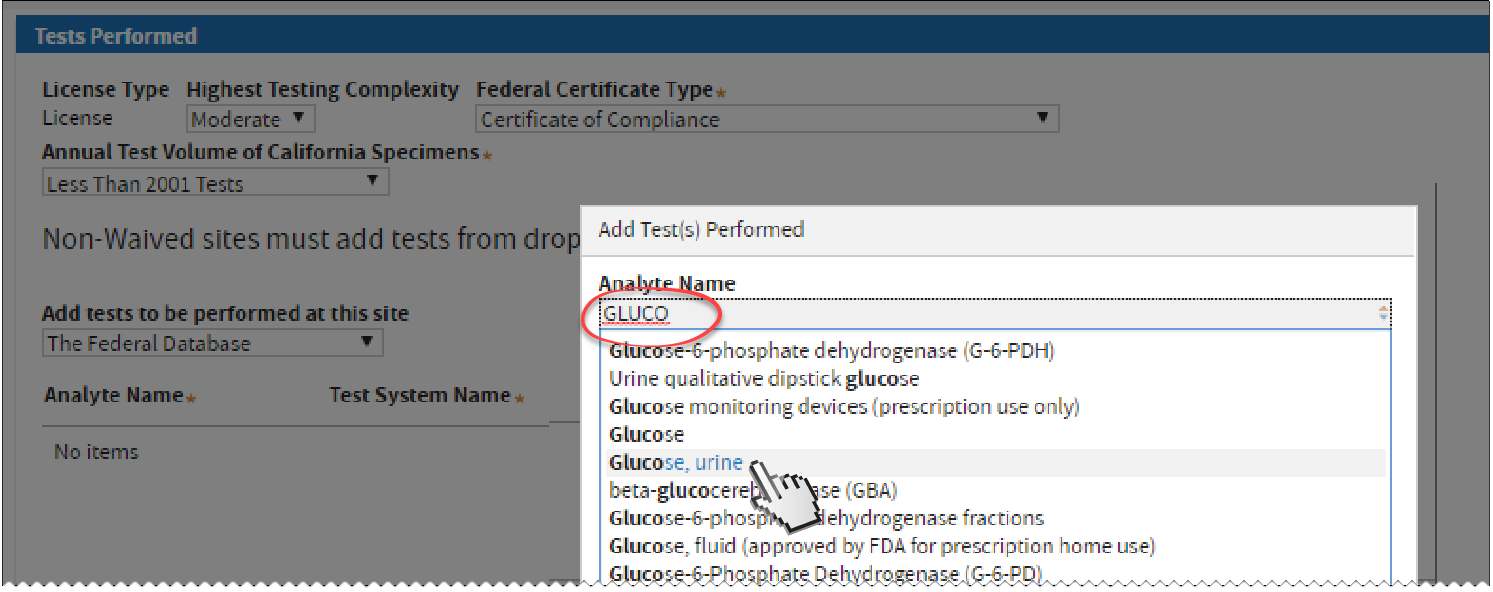
Typing an Analyte Name on the Federal Database will show suggested results. Click one that applies.
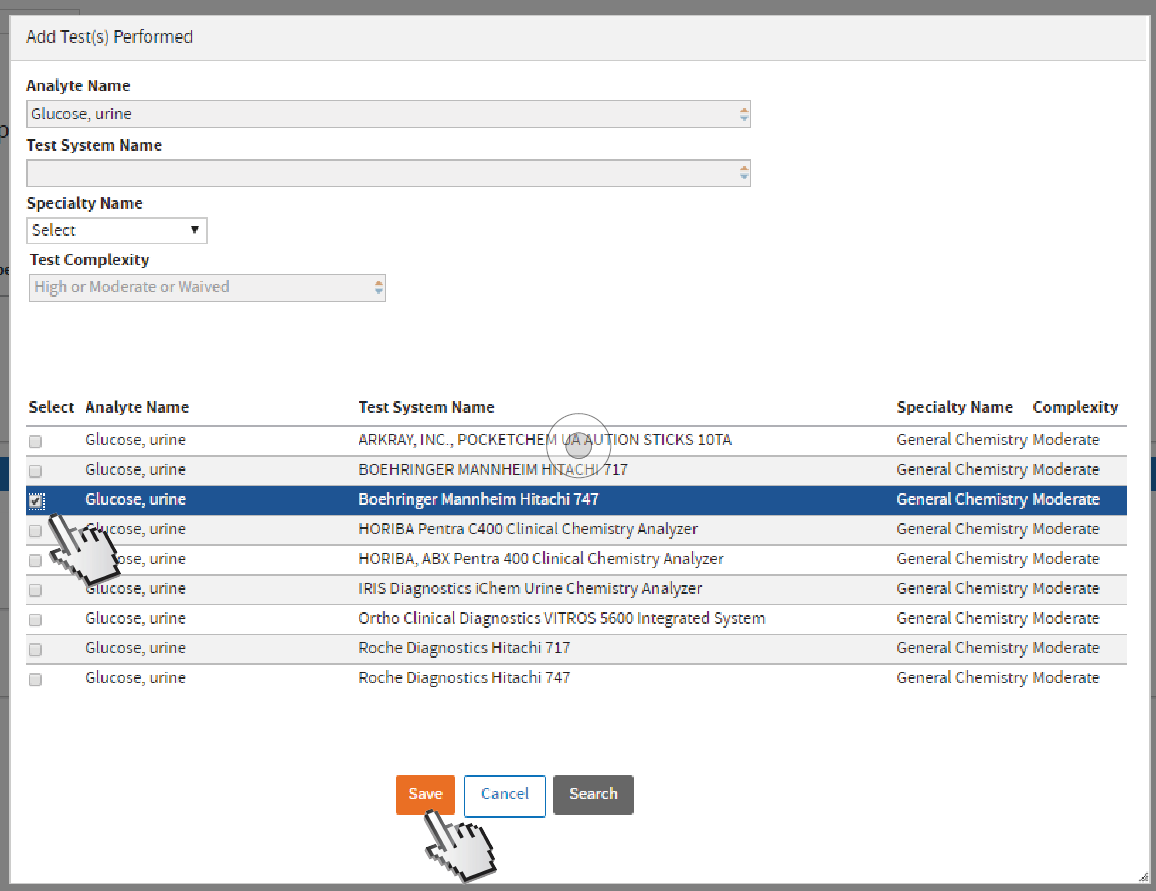
Look for the appropriate
Test System Name and select by clicking its checkbox. Click
Save.
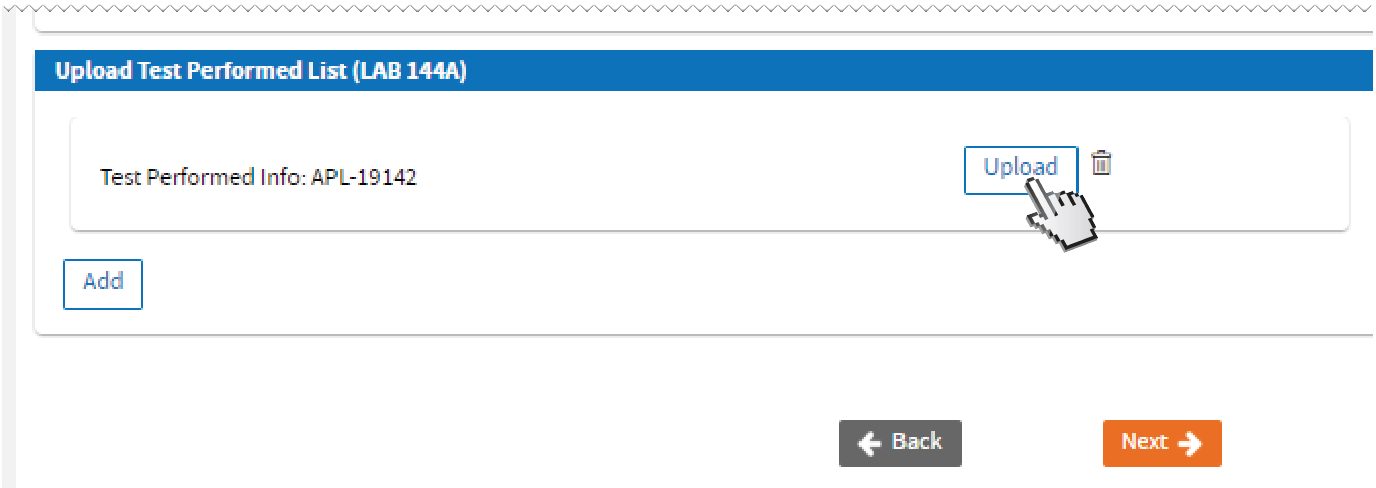
 -OR-
-OR- skip entering tests one by one and upload a completed
 LAB 144A LAB 144A form. Click
Next.
|
 Update Laboratory Testing Personnel
Update Laboratory Testing Personnel
For the
current Laboratory Director, click the
Edit icon to add the required fields.
To
add a Laboratory Director, click
Add Laboratory Director and complete the required fields.
To
remove a Laboratory Director, click the
Trash bin icon on the same row of the item.
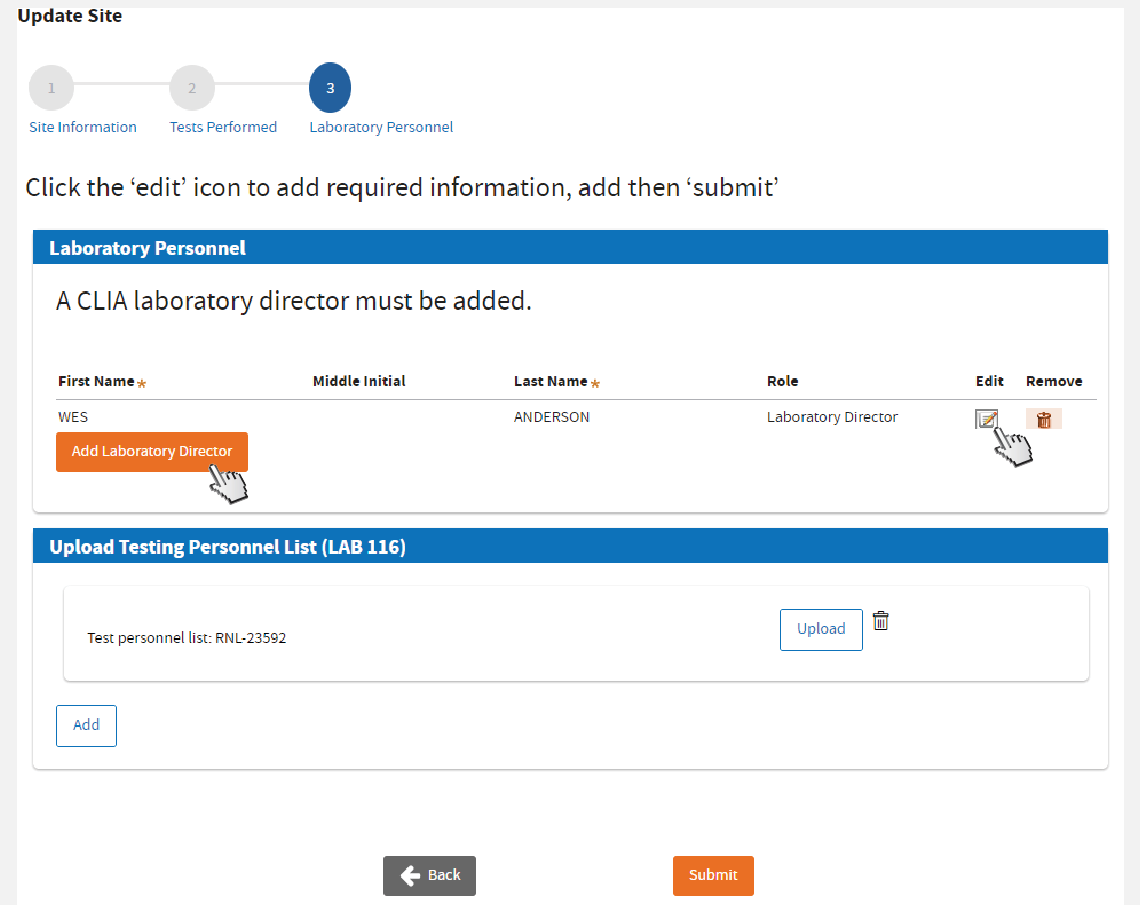 |
Uploading LAB 116 is not required if Certificate Type is “Waiver.”
|
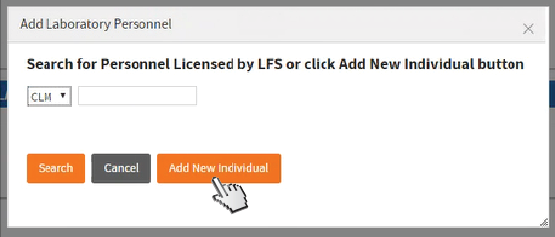
 If you clicked
Add Laboratory Director, a pop-up will appear. Search for
Personnel Licensed by LFS or click
Add New Individual.
If you clicked
Add Laboratory Director, a pop-up will appear. Search for
Personnel Licensed by LFS or click
Add New Individual.
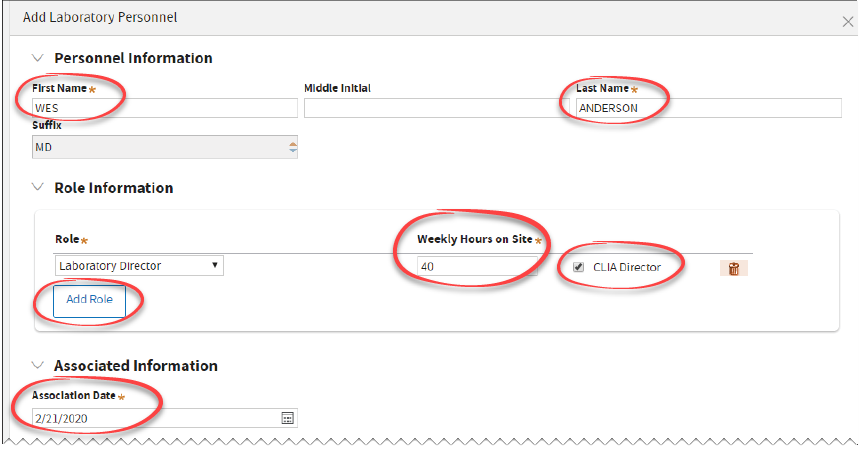
 Enter all the information for this individual and click
Add.
Enter all the information for this individual and click
Add.
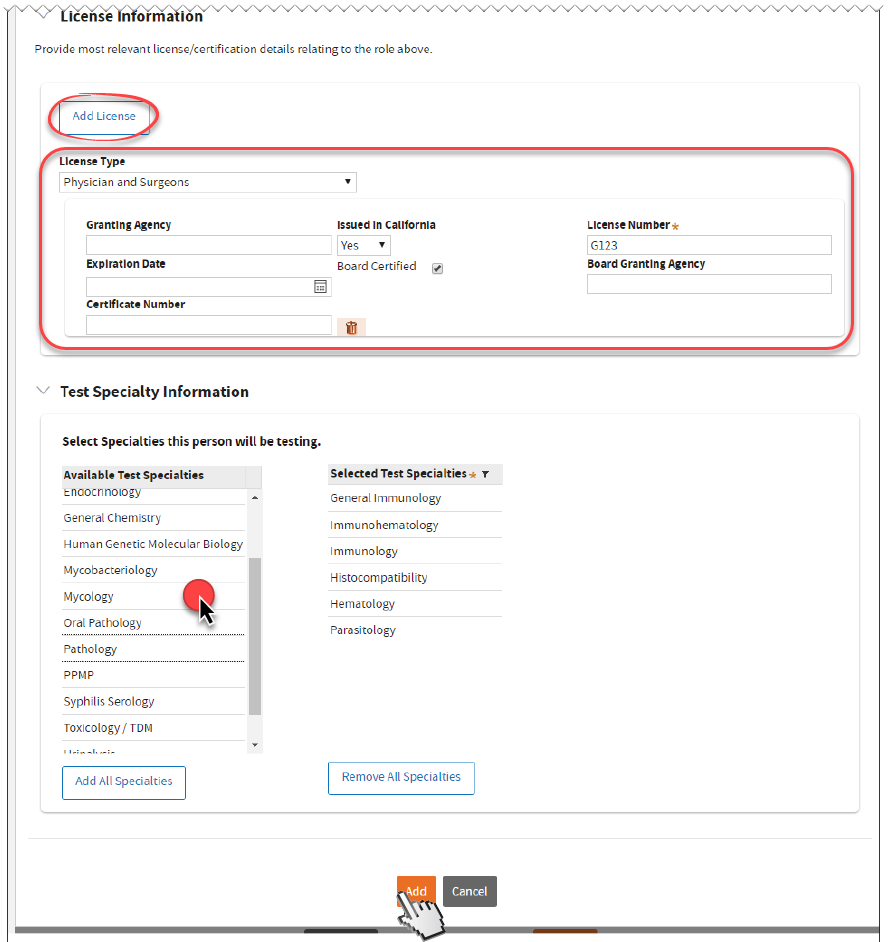
 Upload
Upload LAB 116 form. Uploading LAB 116 is
not required if Certificate Type is
Waiver.
LAB 116 form. Uploading LAB 116 is
not required if Certificate Type is
Waiver.
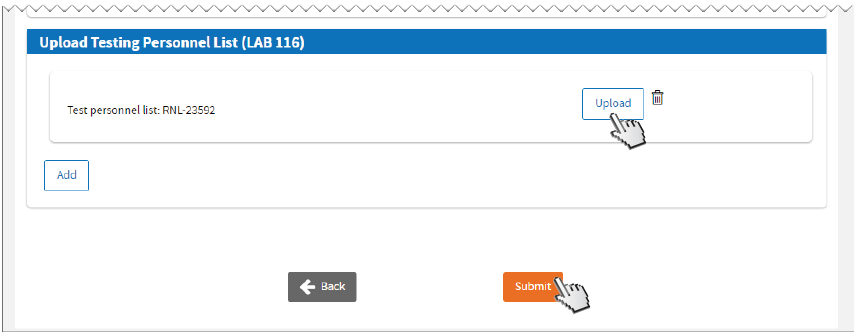
 Click
Close on the confirmation screen.
Click
Close on the confirmation screen.

 You’ve just updated your Testing Site Information. Review and click
Next to proceed.
You’ve just updated your Testing Site Information. Review and click
Next to proceed.
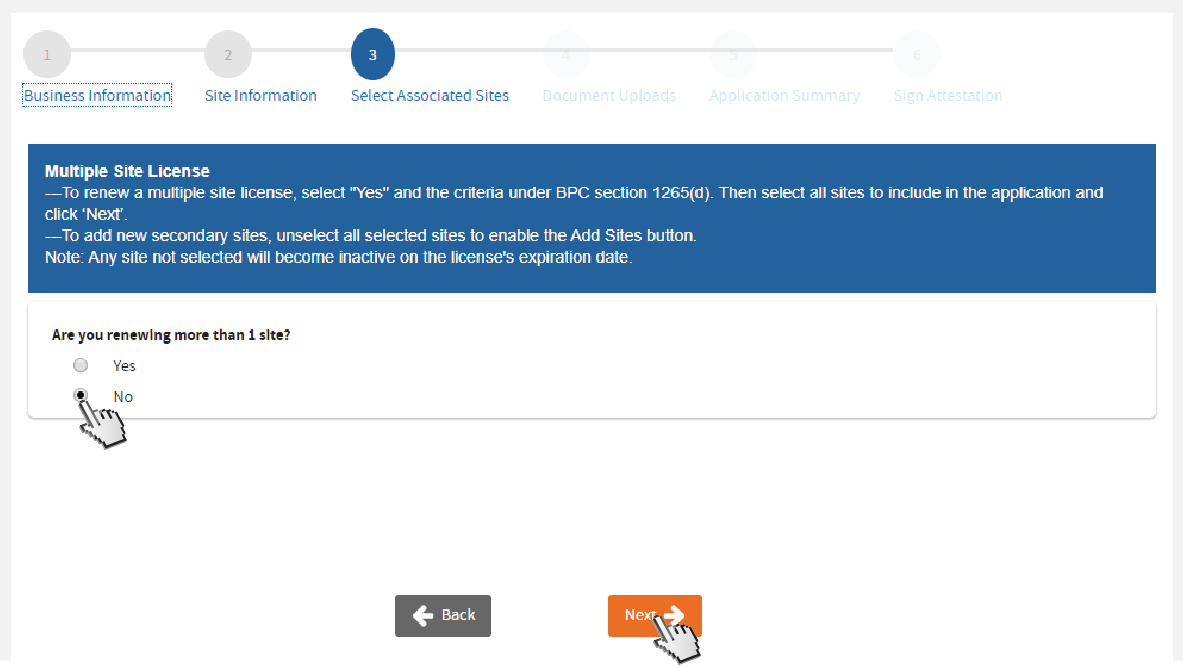
 If renewing a single site, click
No and then
Next.
If renewing a single site, click
No and then
Next.
G. Review, Upload, and Pay
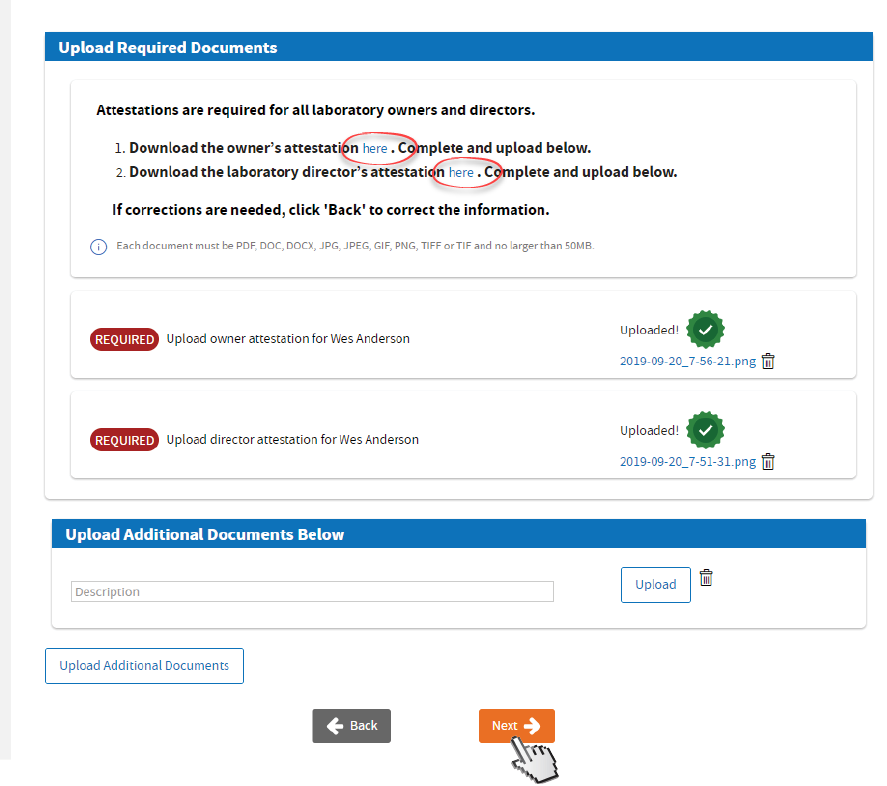
 Click and download the Attestation for Owner (LAB 182) & Laboratory Director (LAB 183).
Click and download the Attestation for Owner (LAB 182) & Laboratory Director (LAB 183).
Complete, scan & upload the required documents. Upload additional documents. Click
Next.
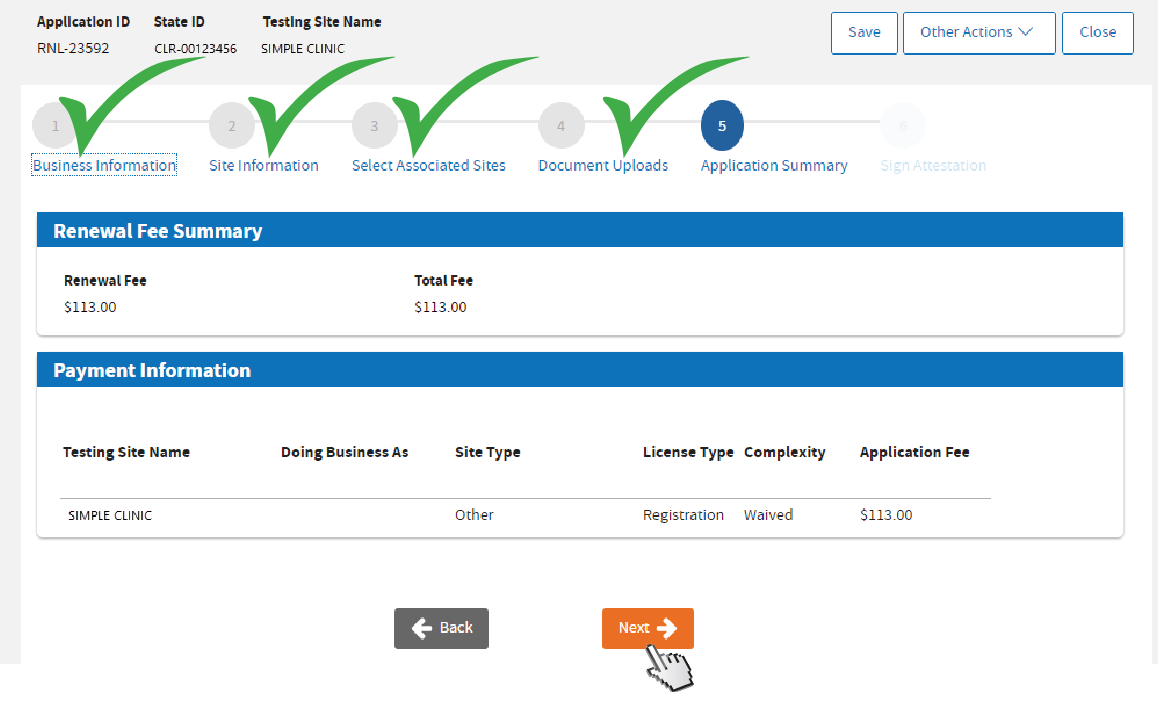
 Review fee and click
Next.
Review fee and click
Next.
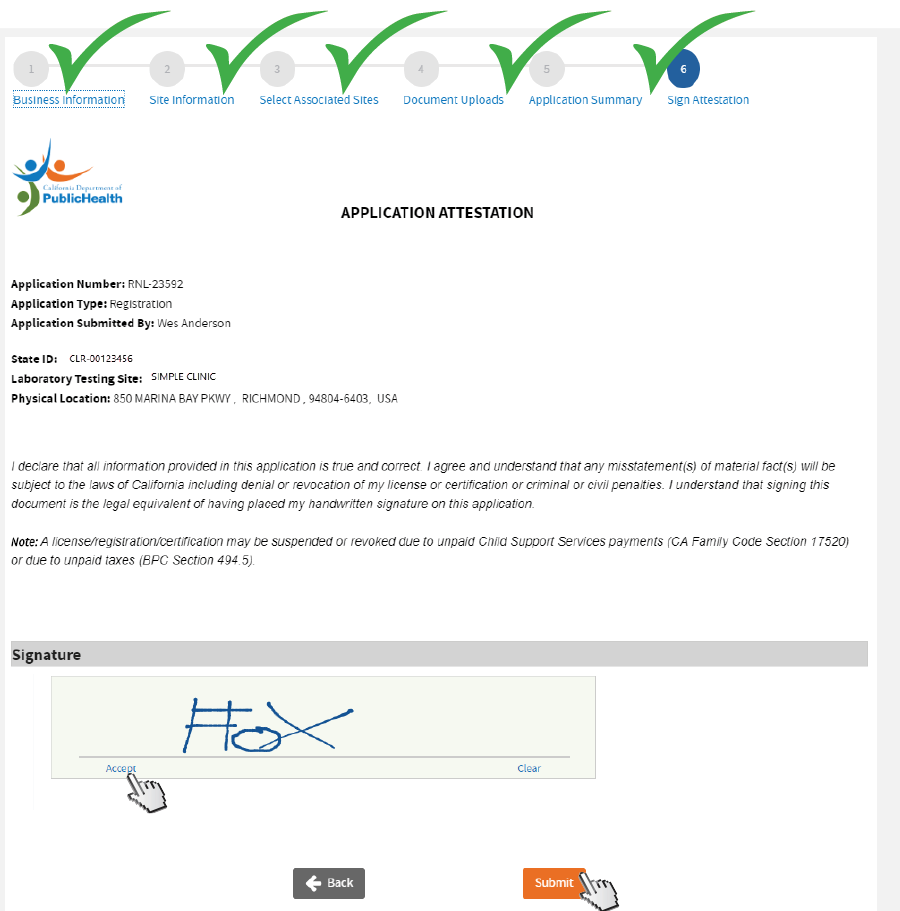
 Review and sign
Application Attestation. Sign with your mouse pointer. Click
Accept before clicking
Submit.
Review and sign
Application Attestation. Sign with your mouse pointer. Click
Accept before clicking
Submit.
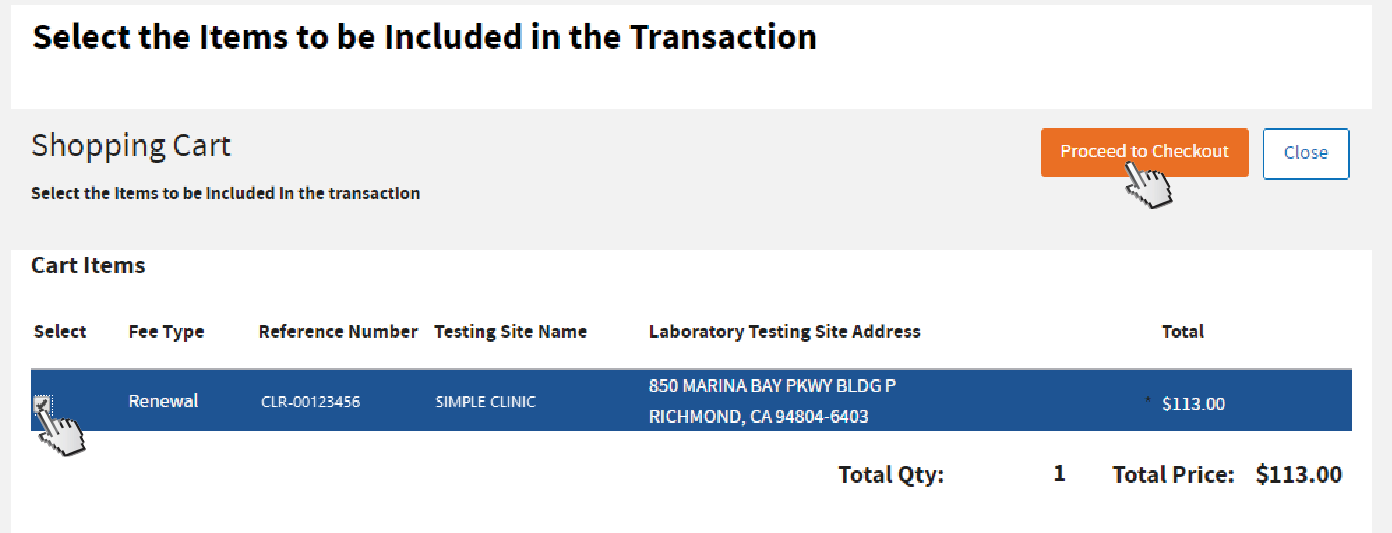
 Click on the
Shopping Cart button.
Click on the
Shopping Cart button.

 Select the testing site, then click
Proceed to Checkout.
Select the testing site, then click
Proceed to Checkout.
 Select your payment method.
Select your payment method.
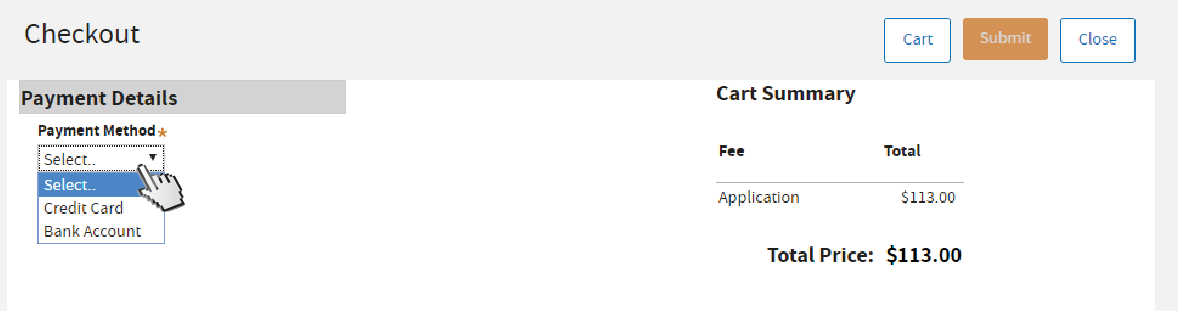
 Fill out all required fields marked with asterisks (*). Read the authorization statement, then
check the box to agree. Click
Submit.
Fill out all required fields marked with asterisks (*). Read the authorization statement, then
check the box to agree. Click
Submit.
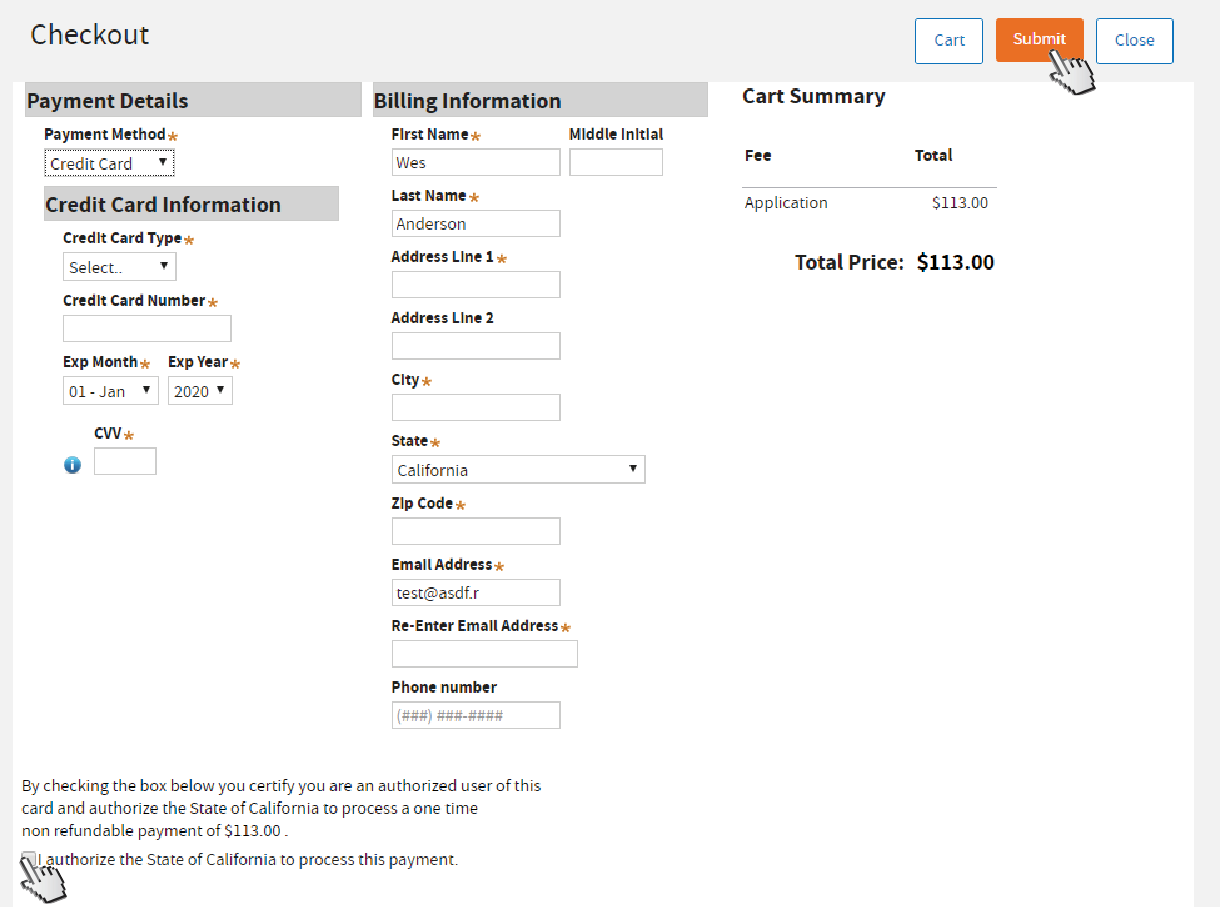
 You have successfully submitted a renewal application. We will review your application and contact you by email if we need more information. Click
Close to go back to your dashboard.
You have successfully submitted a renewal application. We will review your application and contact you by email if we need more information. Click
Close to go back to your dashboard.
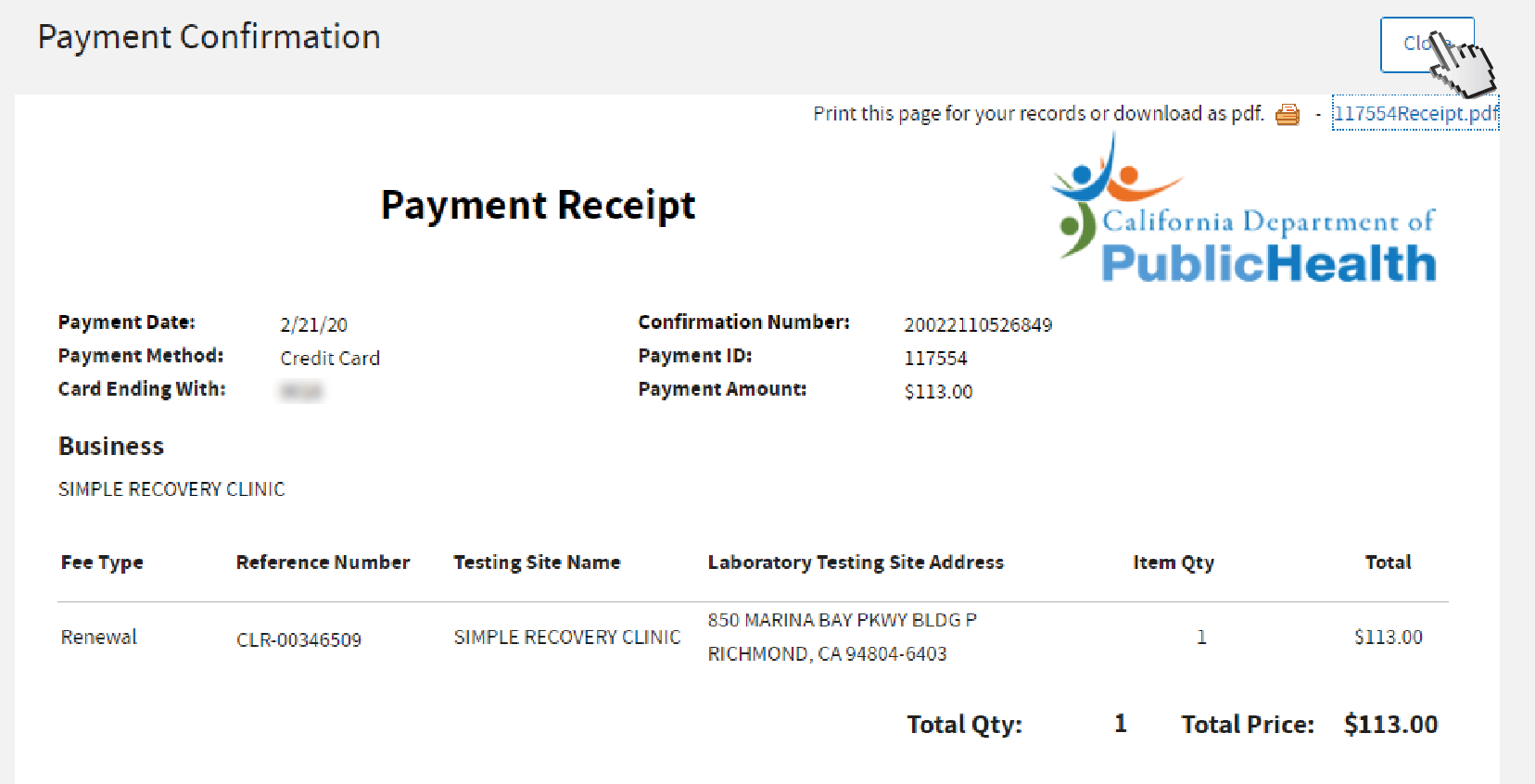
 Click
Refresh. Your dashboard shows the status of your application.
Click
Refresh. Your dashboard shows the status of your application.
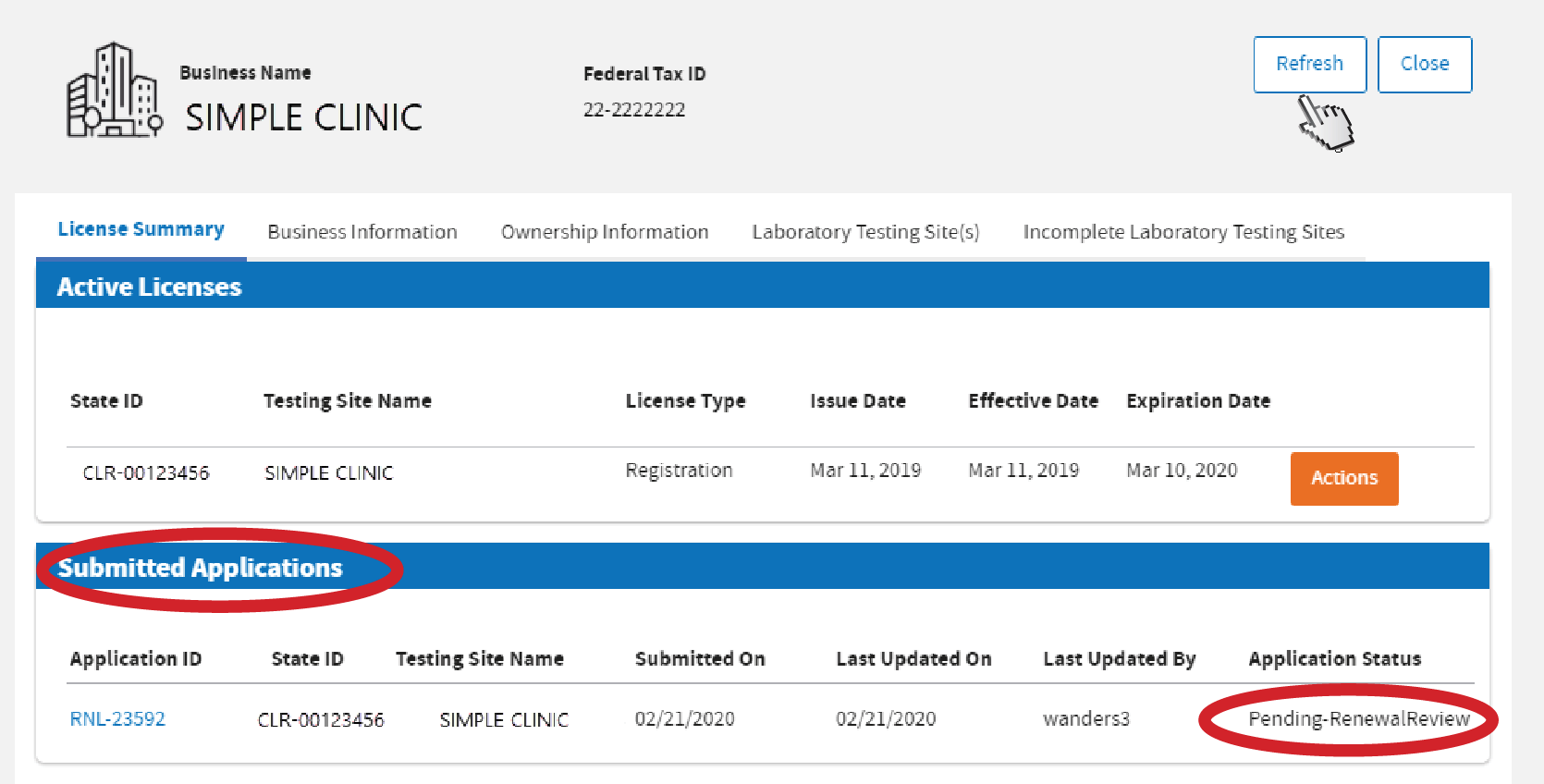
Upon approval, a digital copy of the certificate will be sent to both email addresses you entered in your
Business Information and Testing Site Information.

*Frame not included.
-END-