CDPH and local public health partners are alerting healthcare providers of the continued identification of Candida auris (C. auris) cases in Southern California, and the emergence of C. auris cases linked to hospitals and skilled nursing facilities (SNF) in Southern Nevada since August 2021. Since June 2022, we have identified two cases with reported exposure in Nevada healthcare facilities with known outbreaks.
C. auris containment continues to be an urgent public health priority. Patients and residents who have had prolonged admission in healthcare settings, particularly high-acuity long-term care facilities including long- term acute care hospitals (LTACH) and ventilator-equipped skilled nursing facilities (vSNF), are at highest risk of C. auris and other multidrug-resistant organism (MDRO) colonization and infection. Additionally, patients with long-term admissions in high-acuity ACH units (e.g., ICU and step-down units (SDU)) might also be at an increased risk for C. auris acquisition.
To proactively prevent further spread of C. auris in California, the HAI Program recommends the following updated IPC and containment strategies to healthcare facilities:
Active Surveillance
- Assess C. auris status for all patients and residents upon admission, by reviewing medical records and following up with the transferring facility as necessary.
- Conduct screening through colonization testing for individuals at highest risk for C. auris, whose status is unknown.
- Screen patients transferring from any LTACH regardless of location, SNF ventilator unit in jurisdictions with C. auris transmission,* or other facility with known C. auris transmission, and place on empiric Contact Precautions while awaiting results.†
- Screen patients transferring from any LTACH, ACH, or vSNF in Nevada state, and place on empiric Contact Precautions while awaiting results.†
- Consider screening patients with other known risk factors.‡
- If C. auris is identified on admission, notify the transferring facility and local health department of the patient's status.
Environmental Cleaning and Disinfection
- In LTACHs facility-wide, SNF ventilator units , and ACH high-acuity units (e.g., ICU and SDU), routinely clean and disfinfect surfaces and shared medical equipment using an Environmental Protection Agency (EPA) registered hospital-grade disinfectant with claims against C. auris from List P (www.epa.gov/pesticide-registration/list-p-antimicrobial-products-registered-epa-claims-against-candida-auris).
- If a List P disinfectant is unavailable, a disinfectant from List K (www.epa.gov/pesticide-registration/list-k-epas-registered-antimicrobial-products-effective-against-clostridium) or an appropriately-prepared bleach solution may be used.
- Bleach, and List P and List K disinfectants are also effective against SARS-CoV-2.
In addition, the HAI Program continues to recommend the following routine IPC and containment practices for C. auris:
Infection Prevention and Control
- In ACHs and LTACHs, place any patient with C. auris on Contact precautions, and if possible, in a single room.
- In SNFs, Enhanced Standard Precautions (www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/ESP.aspx) are recommended facility-wide in the absence of C. auris transmission.
- When cohorting patients by COVID-19 status, consider C. auris and other MDRO status during room placement. For example, a patient with both COVID-19 and C. auris can only be placed in the same room as another patient with COVID-19 and C. auris.
- Do NOT reuse or extend use of gloves or gowns (www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html).
- Perform hand hygiene before putting on personal protective equipment (PPE), after removing PPE, and before and after patient contact.
- Regularly monitor healthcare personnel (HCP) adherence to IPC practices (www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/MonitoringAdherenceToHCPracticesThatPreventIn fection.aspx).
- Continue IPC measures for the duration of a C. auris-colonized or -infected patient's admission. There is no 'clearance' for C. auris colonization.
Routine Surveillance
- Identify all Candida isolates from normally sterile sites to the species level; for Candida isolated from non-sterile sites (e.g., urine), consider species-level identification of isolates from patients at highest risk for C. auris.
- Do not rescreen patients previously identified with C. auris; they can remain colonized indefinitely.
Communication
- Communicate a patient's C. auris and other MDRO status to any receiving healthcare facility prior to transfer; use an interfacility transfer form (www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/InterfacilityCommunication.aspx). Receiving facilities should proactively ask about the patient's status if not included in the accompanying medical records.
Antimicrobial Stewardship
- Implement antimicrobial stewardship for broad-spectrum antibacterial and antifungal agents to limit the emergence of C. auris, especially multidrug- or pan-resistant C. auris, and other MDROs.
Reporting Requirements
- Report any cases of C. auris, carbapenemase-producing organisms, or other unusual or highly- resistant organisms to your local health department and the CDPH HAI Program at HAIprogram@cdph.ca.gov.
Public Health Testing
- C. auris identification and confirmatory testing are available at some local public health laboratories, the CDPH Microbial Diseases Laboratory (MDL), and the CDC Antibiotic Resistance Laboratory Network (AR Lab Network).
- Colonization testing (screening) for C. auris is available at no cost through the AR Lab Network.
- These services can be accessed through your local health department in consultation with the CDPH HAI Program by contacting HAIProgram@cdph.ca.gov.
Additional Resources
CDPH C. auris Quicksheet (PDF) (www.cdph.ca.gov/Programs/CHCQ/HAI/CDPH%20Document%20Library/C%20auris%20Quicksheet_Interim_070720_ADA.pdf)
CDC/CDPH C. auris and other MDRO Prevention Webinar Recording (youtu.be/5uIpo7wi6xk) (Opens in YouTube)
CDC/CDPH C. auris and other MDRO Prevention Webinar Slides (PDF) (www.cdph.ca.gov/Programs/CHCQ/HAI/CDPH%20Document%20Library/C_auris_AHR_CDC_CDPHshareWebinarcCombined_ADA_121020.pdf)
CDPH Additional MDRO Resources (www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/AntimicrobialResistanceLandingPage.aspx)
Figure and Table
Figure 1. C. auris Cases in California by Local Health Jurisdiction, January 2020 through June 2022
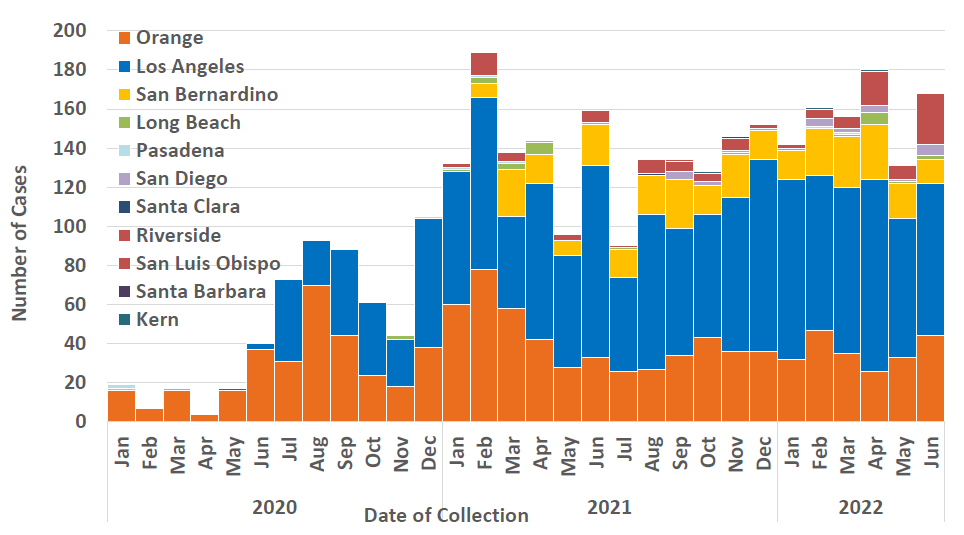
Table 1. C. auris, other MDRO (including C. diff) and COVID-19 Containment, Infection Control Measures
Good hand hygiene – ABHS preferred | X | X | X | Soap & water | X |
| Contact precautions, single room if possible | X | X
| X | X | + respirator, eye protection |
| Thorough environmental cleaning and disinfection | Use List P agent (www.epa.gov/pesticide- registration/list-p- antimicrobial-products- registered-epa-claims- against-candida-auris) List K agent or bleach, OK | X | X | Use List K agent (www.epa.gov/pesticide-registration/list-k-epas- registered-antimicrobial-products-effective-against-clostridium)
| Use List N agent (www.epa.gov/coronavirus/ about-list-n-disinfectants- coronavirus-covid-19-0) List P and K agents OK |
| Routine adherence monitoring | X | X | X | X | X |
Cohorting of patients and HCP
| X | X | X | X | X |
| Lab surveillance | X | X | X | X | X |
| Screening of high-risk contacts | X | X | X |
| X |
ABHS=alcohol-based hand sanitizer; C. diff=Clostridiodes difficile; CRE=Carbapenem-resistant Enterobacterales
* Includes Kern, Los Angeles, Long Beach, Orange, Pasadena, Riverside, San Bernardino, San Diego, San Luis Obispo, and Santa Barbara.
† As exceptions, if a patient has been screened negative at the transferring facility within 24 hours of transfer, the receiving facility may opt to not repeat screening on admission. If a patient has a pending screening test collected within 24 hours of admission, the receiving facility may opt to await those results while placing the patient on empiric Contact Precautions in lieu of rescreening on admission. If there is any doubt regarding the transferring patient's screening status, the receiving facility should screen them.
‡ See CDPH C. auris website (www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/Candida-auris.aspx) under Colonization Testing