A physician and surgeon, hospital, outpatient clinic, and any other facility, individual, or agency providing diagnostic or treatment services to a patient with a neurodegenerative disease shall grant to the department or the authorized representative access to all records that would identify a case of a neurodegenerative disease or would establish characteristics of a neurodegenerative disease, treatment of a neurodegenerative disease, or medical status of any identified patient with a neurodegenerative disease.
Willful failure to grant access to those records shall be punishable by a civil penalty of up to five hundred dollars ($500) each day access is refused. Any civil penalties collected pursuant to this subdivision shall be deposited by the department in the General Fund.
Table 1. Reportable ICD-10 Codes and Their Clinical Descriptions
ICD-10 Code
| Description
|
| G20.A1 | Parkinson's disease without dyskinesia, without mention of fluctuations |
| G20.A2 | Parkinson's disease without dyskinesia, with fluctuations |
| G20.B1 | Parkinson's disease with dyskinesia, without mention of fluctuations |
| G20.B2 | Parkinson's disease with dyskinesia, with fluctuations |
| G20.C | Parkinsonism, unspecified |
| G90.3 | Parkinsonism with neurogenic orthostatic hypotension, Multiple System Atrophy (MSA), MSA-Parkinson (MSA-P), MSACerebella (MSA-C) |
| G35 | Multiple Sclerosis |
| G37.3 | Acute transverse myelitis in demyelinating disease of central nervous system |
| G37.8 | Other specified demyelinating diseases of central nervous system |
| G37.9 | Demyelinating disease of central nervous system, unspecified |
Please Note: Case definitions for Alzheimer's are still under development.
|
Any encounter type can trigger the requirement for reporting, including ambulatory visits, emergency department visits, inpatient hospital stays, non-acute institutional stays, and other outpatient visits. However, encounters are only reportable when (a) the patient has not been previously reported to the CNDR or CPDR or (b) the triggering reportable diagnosis resulted in a change to the medical record (either entering an initial diagnosis or change in diagnosis) during the encounter; thus encounters are not reportable if the qualifying diagnosis is noted on the Problem List but is not applied to the encounter itself. Reportable encounters include face-to-face visit as well as certain non-face-to-face visits (telemedicine, telephone, online/e-visits) that include the specific diagnoses addressed. However, ancillary encounters (e.g., lab, imaging, cardio-pulmonary, and therapies), are excluded from the triggering encounter list. Inpatient encounters can rely on diagnostic coding done for hospital billing purposes to trigger reportability.
Figure 1. Flow chart of Reportable Neurodegenerative Disease Cases
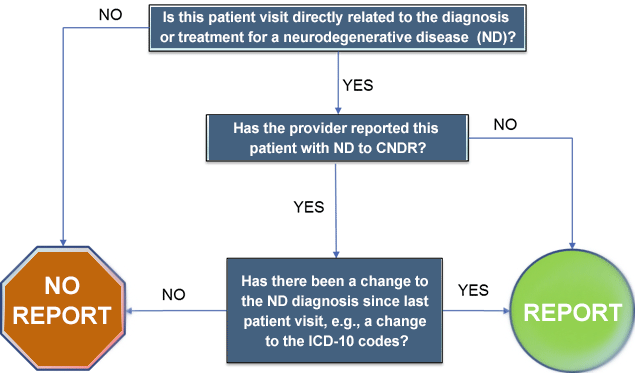
Reportable data elements are identified in the Neurodegenerative Disease Guide to Reporting (PDF). Below are some of the data elements that will be required to report for manual reporting for a full list of the data elements please review Appendix 1. Table of Data Elements for Manual Reporting to the California Neurodegenerative Disease Registry (PDF).
- Patient ID:
- Name (Last, First, M)
- Date of Birth
- Sex (Gender)
- Address
- and more
- Patient Demographics:
- Patient Visit Information:
- Attending Doctor
- Consulting Doctor
- and more
- Physician Identifiers (Primary):
- Physician Name
- Author NPI - Physician ID
- and more
- and more
Below are some of the data elements that will be required to report for electronic reporting for a full list of the data elements please review Appendix 2. Table of Data Elements for Electronic Reporting to the California Neurodegenerative Disease Registry (PDF).
- Facility ID:
- Reporting Facility Name
- Reporting Facility ID
- Facility Address
- and more
- Software ID – If using HL-7:
- Software Vender Organization
- Software Version or Release Number
- and more
- Patient ID:
- Name
- Date of Birth
- Sex (Gender)
- and more
- Patient Demographics:
- and more
Regardless of the reporting modality (manual entry via web portal or submission via electronic interface), timing of reporting is based on the calendar quarter during which the encounter occurred. For the first quarter of the mandate (i.e., patient encounters occurring between July 1, 2023-March 31, 2024) encounters must be reported within six months following the end of the quarter (e.g. September 30, 2023). Beginning August 19, 2023, the deadline for data submissions is three months following the end of the quarter. Frequency of data submission may be determined by the reporting physician or facility, but all data must be received by the deadlines outlined below (See Table 2). For inpatient encounters, the discharge date may be used as the date of the encounter.
Table 2. Data Collection Timeframe and Case Submission Deadlines
| July – September |
December** |
October – December
|
March |
January – March
|
June
|
| April – June |
September
|
* For inpatient settings, the discharge date may be used as the date of the patient encounter.
**The first reporting period (CPDR: Ongoing, CNDR: July-September 2023 ) has a six-month deadline (i.e., data is due to the CNDR by 3/31/2024); All subsequent reporting periods have three-month deadlines.
All data elements listed in the Neurodegenerative Disease Guide to Reporting (pdf) Appendix 1 and Appendix 2 are required or required if available. If a data element is required, it must be transmitted with a value other than empty, blank, or null, or the record will not be accepted. For a data element that is required if available, it must be sent when a known value is available in the sending system. However, if a data element has an allowable code for "unknown", then that code should be transmitted for that element instead of an empty value.
Please register your organization's intent to report before the July 1, 2023, deadline!
Per requested registration information, please determine whether you can report automatically with your EHR. If your facility cannot interface directly, you can use the Direct Data Entry Web Portal.
Steps to Start Reporting:
Step 1: Register to Report
First-time users will need to REGISTER by completing the CNDR Electronic and Manual Reporting Registration Form (pdf) and submit it to CDSRBHelp@cdph.ca.gov. If you will be reporting via the Web Portal (Manual Reporting), please also complete the CalREDIE Neurodegenerative Disease Reporter Account Authorization Form (pdf) and submit it to CDSRBHelp@cdph.ca.gov.
Facilities are required to register for reporting on behalf of represented neurologist within an organization. Registration information includes Lead physician contact, administrative/business management contact, technical interface contact, EHR vendor information for the purpose of providing a certificate for submission to the web service where applicable.
If you are currently reporting Parkinson's data and your intention is to add reporting of MS data, please email us at CDSRBHelp@cdph.ca.gov to let us know.
Step 2: Onboarding – If your facility has registered what are the next steps.
Pick a Transmission Method:
Submit Data via Direct Data Entry Web Portal:
Electronic Interface Options: If your facility will be reporting utilizing an electronic interface between your EHR system and CNDR, the next steps are as follows:
- Contact CDSRBHelp@cdph.ca.gov to discuss options for secure connection methods and transmission format standards
- Visit the Self-Testing Portal:
- Here you can download a sample HL7 Message that your team can use to format your message.
- Once your message passes validation from the portal, a reportable case trigger mechanism from your EHR needs to be identified and established.
- Your facility has passed validation, and a reportable case identification mechanism has been identified.
- The next step is to begin acceptance testing by sending test messages to the state.
- Request credentials for your message submission method (SOAP, SFTP, FHIR) by sending an email to CDSRBhelp@cdph.ca.gov.
- Once your message submissions have been validated your facility will be moved to production for ongoing submissions to the central registry.
- If you are currently submitting Parkinson's data through the electronic interface and you will be reporting your MS data via the same method, please email us at CDSRBHelp@cdph.ca.gov to let us know.
All LIPs and EHR vendors should reference the Neurodegenerative Disease Guide to Reporting (pdf) and review the reporting requirements to ensure the required data is being transmitted.
To maintain high standards in reporting accuracy, please visit our Resources for Reporting section. All healthcare facilities and providers should reference the Neurodegenerative Disease Guide to Reporting(pdf) and review the reporting requirements to ensure the required data is being transmitted.