Related Materials: | CDC Health Alert Network (HAN) | California Health Alert Network (CAHAN) |
Mpox landing page | Mpox Q&A |
CDPH Information for Health Care Providers |
CDPH Letter for CA Healthcare Providers for Severe Mpox (PDF)
Updated as of June 14, 2023:
Background and Summary
The California Department of Public Health (CDPH) continues to work with local health departments (LHDs) and California health care providers on the ongoing mpox situation impacting the United States and other countries not usually endemic for mpox. Investigations in several countries and the U.S., including in California, suggest that transmission occurs most often through close personal contact and that clinical case presentations have not always been characteristic of classic mpox infections.
Evaluation for Suspected Mpox Cases
Mpox spreads between people primarily through direct contact with infectious lesions or mucosal surfaces (CDC Science Brief: Detection and Transmission). It can also be spread by respiratory secretions during prolonged face-to-face contact (CDC Mpox Response: Transmission).
Mpox can spread during intimate contact between people, including during sex and from close contact activities like kissing, cuddling, or touching parts of the body with mpox lesions. At this time, it is not known definitively if mpox can spread through urine, feces, semen, or vaginal fluids (CDC Science Brief: Detection and Transmission).
A few infections have resulted from injuries with a sharp instrument used in a clinical situation to sample skin lesions,
a practice that CDC recommends against. Mpox has also been transmitted through skin piercing, tattooing, and occupationally to healthcare workers in the absence of a known sharps exposure; the precise means of transmission for these cases still remain unknown.
A patient should be considered a suspect case if they have a new characteristic rash OR if they have risk factors for mpox exposure and clinical suspicion for mpox.
Features of the typical disease course are shown below:
Incubation Period
| ~3 days – 3 weeks
| May be contagious*
| Monitor for symptoms and avoid sexual contact**
|
Prodrome | 1 – 4 days | Contagious | Isolate |
Rash Stage | 2 – 4 weeks | Contagious | Isolate |
Recovery | 4 weeks or longer | ***
| ***
|
*Current evidence indicates all persons are infectious with the onset of illness (i.e., rash or other related symptoms), however, some people can also transmit the virus to others up to four days before they develop signs or symptoms (i.e., while presymptomatic). At this time, there is no evidence that persons who are infected but eventually clear the infection without developing illness (i.e., asymptomatically infected) have transmitted the mpox virus to
others. Knowledge regarding the means by which mpox virus spreads is evolving and is subject to change.
**Contacts of probable and confirmed cases should be monitored, or should self-monitor daily, for any sign or symptom during a period of 21 days from last contact. Quarantine or exclusion from work are not necessary while no symptoms are evident but known contacts should
avoid sexual contact with others during the 21-day monitoring period, regardless of any symptoms.
***A person is contagious until after all the scabs on the skin have fallen off and a fresh layer of skin has formed. The infectious and recovery period may be longer in severe cases.
Physical Exam
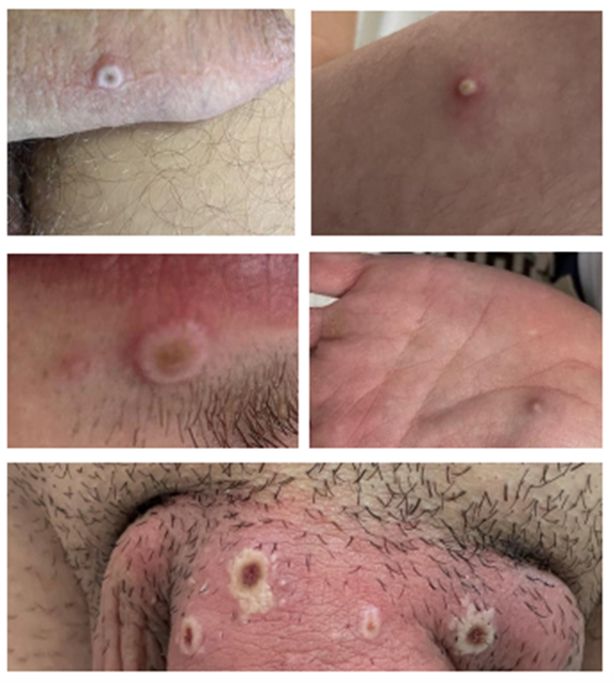
The rash associated with mpox classically involves vesicles or pustules that are deep-seated, firm or hard, and well-circumscribed. The lesions may umbilicate or become confluent and progress over time to scabs, however, presentations in this outbreak have not always been classic. Patients have experienced rashes without prodromal symptoms, rashes at different stages within an affected area, or rashes that do not involve the face or extremities but only the genital and/or perianal areas.
Clinicians should perform a thorough skin and mucosal (e.g., anal, vaginal, oral) examination for the characteristic vesicular or pustular rash of mpox; this allows for detection of lesions of which the patient may not have previously been aware.
Figure 1: Examples of mpox lesions, from CDC Health Alert Network 6/14/2022
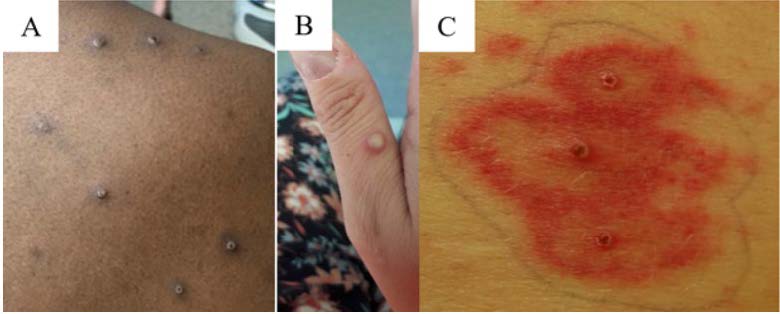
Figure 2: Photo credit – General Hospital University of Malaga
Clinical Decision Guide
| | |
1. Did the patient have a prodrome | Yes: Recent cases have presented without an obvious prodrome. However, a patient with a strong epidemiologic link PLUS prodromal symptoms might increase suspicion of mpox. Notably lymphadenopathy is a distinguishing feature of mpox. | No: Recent cases have presented without an obvious prodrome. A patient with an epidemiologic link without prodromal symptoms might decrease suspicion of mpox. Close monitoring should occur for development of a rash or other symptoms. |
2. Did the patient develop a rash? | Yes: Most cases to date in California have developed a rash at some point in their course. | No: Some cases have developed anorectal pain, tenesmus or bleeding, but these were from non-visible perianal lesions. |
| Uncertain: Classically, mpox rashes have started in the face and extremities then spread to rest of body. In recent cases, rash has often begun in mucosal areas (e.g., genital, perianal, oral mucosa) and in some patients, the lesions have been scattered or localized to a specific body site rather than diffuse and have not involved the face or extremities. | Uncertain: Classically, mpox rashes have started in the face and extremities then spread to rest of body. In recent cases, rash has often begun in mucosal areas (e.g., genital, perianal, oral mucosa) and in some patients, the lesions have been scattered or localized to a specific body site rather than diffuse and have not involved the face or extremities. |
4. What is the rash appearance? | Deep-seated and well-circumscribed lesions, often with central umbilication. Lesions progress through specific sequential stages, sometimes rapidly—macules, papules, vesicles, pustules, and scabs. Additional information can be found at the mpox CDC page. | Other presentations of rashes and rashes that do not progress. Remember, rashes in certain stages can be mistaken for other common rash etiologies, including sexually transmitted infections (STIs) such as syphilis, herpes, etc. |
5. Is the stage of rash consistent within each body part? | Uncertain: Classically, lesions on each part of the body evolved at the same stage; however, recent cases have had rashes at different stages of progression in the same part of the body. | Uncertain: Classically, lesions on each part of the body are at the same stage; however, recent cases have had rashes at different stages of progression in the same part of the body. |
| Yes: Mpox rash is sometimes very painful (or pruritic) and is often a reason people seek evaluation and/or treatment. | No: Rashes such as those associated with HSV can be painful however other STIs such as syphilis are not typically painful. |
7. Did the patient test positive for other rash etiology? | No: Negative test for other etiologies that cause rashes that appear similar to mpox (e.g., VZV, HSV, syphilis) does not rule out mpox entirely. Coinfections with other STIs have been seen with mpox. | Yes: Positive test for other rash etiology, especially one that cause rashes that appear similar to mpox. Coinfections with STIs, particularly syphilis, have occurred in recent cases, so a positive test does not rule out mpox. |
8. Was there contact with a known or suspect mpox case? | -
Contact with lesions or bodily fluids
-
Sexual contacts
-
Household contacts
-
Prolonged (3+ hours) unmasked contact within six feet
| -
Masked contact within six feet
-
Contact with lesions/bodily fluids while wearing PPE
-
Shared airspace contact while at least six feet apart
|
9. Did the patient recently participate in parties or gatherings involving sex, especially with multiple sex partners? Or did the patient participate in intimate contact at venues where there is sex on premises such as bathhouses or saunas? | Yes: There have been a number of cases and contacts that were associated with sex or extended physical contact in sex-related events, in bathhouses/saunas, and/or with multiple sex partners. | No: No participation or contact with someone who has participated in these activities or attended these venues/events is less suggestive of mpox. |
10. Is the patient part of a social group known to have high mpox incidence or vulnerability? | Yes: The majority of cases seen in this outbreak have been in men or transgender persons who have sex with men, however anyone can get mpox. | No: No known linkage to a more vulnerable group or any reported high-risk social or sexual behaviors would be less suggestive of mpox. |
*While some of the listed factors more strongly suggest an underlying mpox etiology, no one answer is absolute in determining whether to suspect mpox; instead, the collective responses and overall clinical picture should be considered.
Clinical Considerations for Mpox in Specific Patient Populations
-
People with HIV
- People with advanced and uncontrolled HIV can be at a higher risk of severe or prolonged mpox disease.
- Co-infections with the mpox virus and STIs, including HIV, have been reported, therefore a broad approach to testing is recommended.
-
Providing tecovirimat promptly should be considered for use in patients with mpox who have advanced or poorly controlled HIV, as they may be
at high risk for severe disease.
- More information on "Severe Manifestations of Mpox Among People who are Immunocompromised Due to HIV or Other conditions" is found at CDC HAN and on
CDC MMWR.
-
People who are Pregnant or Breast/Chestfeeding
- Close
monitoring for pregnancy complications is important, and the decision to monitor and/or treat a pregnant person as an outpatient or in the inpatient setting should be on a case-by-case basis.
- Treatment for mpox virus should be offered, when indicated, to people who are pregnant, recently pregnant, or breast/chestfeeding.
- Pregnant, recently pregnant, and breast/chestfeeding people should be prioritized for medical treatment if needed.
-
Ocular Involvement
- Involvement of the eyes can be a vision-threatening condition and should be treated urgently.
- Patients should be instructed to avoid touching their eyes and practice hand hygiene while infected.
- Ophthalmology should be involved early in care and systemic antiviral therapy should be considered for all patients with severe mpox, which includes ocular manifestations.
-
Children and Adolescents
- Mpox should be considered when children or adolescents present with a rash consistent with mpox, especially if there is an epidemiologic risk factor.
- Children and adolescents with close contact to people with suspected, probable, or confirmed mpox may be eligible for post-exposure prophylaxis (PEP)
- Treatment should be considered on a case-by-case basis.
-
Severe Manifestations of Mpox
Next Steps
- If you suspect mpox in a patient and need to collect a specimen, refer to the CDC guidance for the preparation and collection of mpox virus specimens, Preparation and Collection of Specimens.
- Importantly, any patient who is a suspect case should be counseled to immediately implement appropriate transmission precautions, including isolation, while waiting for test results.
- Diagnostic testing for mpox, HIV, and other STIs is highly encouraged in every sexually active person who is a suspected mpox case.
- If a patient is confirmed as a case, the CDC's Guidance for Isolation and Infection Control at Home and Guidance for Isolation and Prevention Practices for People with Mpox and CDPH's Home Isolation Guidance provide direction on how people can protect themselves, their household members, and their communities.
- All patients who test positive for mpox are strongly recommended to have HIV testing, as most cases of severe disease and mortality have been linked to undiagnosed and/or untreated HIV.
- Although, it is still unknown if mpox can be transmitted via genital secretions; patients should be counseled on use of condoms during sexual activities. Research is ongoing regarding how long people should use condoms after recovering from mpox, but some countries are recommending a minimum of 8 weeks of condom use after recovery (with other health care groups, such as the World Health Organization, recommending condom use for up to 12 weeks following recovery).
- Health care providers must report cases of patients meeting the definition of a confirmed or probable case (Case Definitions† for Use in the 2022 Mpox Response) to their LHD, as this was added to the list of required reportable diseases and conditions in 2022.
Vaccination for Mpox
The JYNNEOS vaccine is approved by the U.S. Food and Drug Administration (FDA) to prevent both mpox and smallpox. CDPH recommends that those who may be at risk for — or seek additional protection from — mpox infection, as defined within this guidance, be vaccinated against mpox.
Vaccine providers can offer vaccine to any patient who MAY be at risk as there is currently adequate vaccine supply and there are no longer "eligibility" criteria.
Persons who request vaccination should receive it without having to attest to specific risk factors.
Vaccination efforts should be prioritized for:
- Anyone living with human immunodeficiency virus (HIV). It is recommended that additional efforts be made to reach those with a CD4 count <350/mm3, an unsuppressed HIV viral load, or an opportunistic infection, due to increased risk for complications of mpox
- Any man or trans person who has sex with men or trans persons
- People who use or are eligible for HIV PrEP
- Sex workers
- Sexual partners of the above groups
- People who have had direct skin-to-skin contact with one or more people AND who know others in their community who have had mpox infection
- People who have been diagnosed with a bacterial sexually transmitted infection (e.g., chlamydia, gonorrhea, syphilis) in the past 3 months
- People who anticipate experiencing the above risks
The JYNNEOS vaccine is approved as a series of two doses given 28 days apart. The JYNNEOS vaccine can be given via two methods:
The standard method is a subcutaneous injection which is a shot given beneath the skin in the upper arm. This method has been approved for people 18 years or older and is also authorized under an Emergency Use Authorization (EUA) for people younger than 18 years of age.
- Under new guidelines from the FDA and CDC, the vaccine can also be given through
intradermal injection, in the skin layer underneath the epidermis (which is the upper skin layer), for people 18 years or older. Intradermal injection is typically given in the forearm and requires a smaller amount of vaccine than the subcutaneous injection to create a similar immune response. Intradermal injection can also be given in the upper arm or on the back below the shoulder blade.
Public health jurisdictions and healthcare providers have the flexibility to offer the intradermal or subcutaneous regimen based on balancing optimal vaccine use and acceptance, feasibility of administration, and available vaccine supply.
People of any age with a history of developing keloid scars, and individuals younger than 18 years of age, should receive the vaccine via the subcutaneous route.
CDC recommends that people receive two JYNNEOS doses four weeks apart.
CDPH encourages healthcare providers to consider outreach efforts focused on improving vaccination rates in
groups over-represented in cases. Healthcare providers should utilize all available tools and outreach messaging platforms to reach out to these populations, encourage vaccination and a return for second doses, and be flexible on vaccination routes to overcome barriers.
The use of clear messaging and inclusive language are important factors in improving outreach and education, following vaccination rates.
The CDPH vaccine page and JYNNEOS Q&A have additional information and guidance about the vaccine. Contact your LHD for more information in ordering the vaccine.
Treatment
- Health care providers seeing patients with suspected or confirmed mpox infection should provide supportive care and treatment of symptoms. This may include medicines or other clinical interventions to control itching, nausea, vomiting, and pain. For additional information on supportive care, please see CDPH's Supportive Care Guidance.
-
For high-risk patient groups or severe infections, healthcare providers should consider promptly treating high-risk suspect or confirmed cases with tecovirimat (TPOXX), an antiviral medication available through an expanded access Investigational New Drug (EA-IND) protocol for the treatment of mpox infection.
- Antiviral treatment of mpox infection should be considered for patients with severe infection, illness complications, and risk factors for progression to severe infection (children younger than 1 year of age, pregnant or breast/chestfeeding individuals, immunocompromised individuals, or those with a history of atopic dermatitis, eczema, or other conditions affecting skin integrity). Information on antiviral medications for the treatment of mpox infection can be found at CDC Treatment Information and
CDPH Mpox Tecovirimat Treatment Information for Providers.
- In patients with persistent or progressive mpox after completing 14 days of tecovirimat, testing for tecovirimat resistance and pharmacokinetics for public health surveillance purposes should be obtained. Tecovirimat treatment can be extended on a day-by-day basis beyond the standard 14-day course based on clinical course.
- Review the CDC's additional considerations for
Managing Mpox in Patients Receiving Therapeutics and for managing
Severe Manifestations of Mpox which may include concurrent administration of cidofovir, brincidofovir, and vaccinia immune globulin intravenous (VIGIV) in certain patients with (or at high risk for) severe mpox.
Contact your LHD if you need information about sites where you can refer your patient for treatment. For additional information regarding tecovirimat treatment, refer to
CDPH's treatment guidance for providers.
Additional Information and Resources