Background
The California Department of Food and Agriculture (CDFA) has been managing an outbreak of avian influenza A in dairy cattle in California since late August 2024, and outbreaks in poultry since 2022. On 10/3/24, CDPH announced two positive avian influenza A infections in California residents exposed to infected dairy cattle. The risk to the general public remains low. These infections were detected through public health implementation of CDC’s recommended monitoring and testing strategies in exposed persons and in close partnership with the CDFA. The two persons are workers at separate dairy facilities in the Central Valley that are experiencing avian influenza A outbreaks among dairy cattle. Both persons reported symptoms of conjunctivitis, and one reported fever as well. Both persons were prescribed influenza antiviral medication and are following guidance to isolate from others. Local public health is following up to identify close contacts, offer influenza antivirals to close contacts as post-exposure prophylaxis and ensure they are monitored for symptoms. Investigation is ongoing.
Seasonal activity for RSV, influenza, and SARS-CoV2 is expected to increase in the coming weeks and months,
and
forecasting for this winter suggests similar or lower combined peak hospitalization burden for COVID-19, influenza, and respiratory syncytial virus (RSV) compared with last year. Scenario modeling suggests a later influenza season compared with last year.
CDPH urges healthcare providers to prepare for increases in respiratory viral activity in advance to maximize prevention and reduce severe disease and healthcare impacts. Providers may stay informed by accessing respiratory activity and surveillance data from
CDC and
CDPH. COVID-19, influenza, and RSV pose the greatest risk for infants and older adults, especially
older adults with chronic medical conditions and those who are immunocompromised. Providers should identify patients at high risk and prioritize prevention and therapeutic strategies for these populations.
Disease-Specific Guidance
SARS-CoV-2
The following actions are recommended for COVID-19 prevention and treatment:
-
Children 6 months through 4 years require a 2 (Moderna)- or 3 (Pfizer)-dose initial series.
Antibody pre-exposure prophylaxis (PrEP):
Pemivibart (Pemgarda) is authorized for use as PrEP in moderately-to-severely immunocompromised individuals who may not mount an adequate immune response to vaccination. Of note, the use of this medication may be limited when the national frequencies of variants with reduced susceptibility to Pemgarda is less than or equal to 90%. PrEP with Pemgarda is not a substitute for vaccination and all individuals who can receive vaccination should do so.
Therapeutics: Treatment
as soon as possible
with COVID-19 antivirals decreases risk of serious illness, hospitalization, and death. Healthcare providers are recommended to evaluate symptomatic patients who are
eligible for treatment e.g. those at higher risk for severe illness. Paxlovid is the first line therapy for mild to moderate COVID-19 in the outpatient setting. The CDPH COVID-19 Treatments and CDC
COVID-19 Treatments webpages contain more information about therapeutic treatment.
Influenza
The following actions are recommended for influenza prevention and treatment:
-
Adults 65 years and older should preferentially receive any one of the following: higher dose, recombinant, or adjuvanted flu vaccines. If none of these three vaccines is available at an opportunity for vaccine administration, then any other age-appropriate influenza vaccine should be used.
-
New: All U.S. influenza vaccines are now trivalent, containing A(H1N1), A(H3N2), and B(Victoria) influenza strains.
-
Therapeutics:
Influenza antiviral treatment is recommended as soon as possible (ideally <48 hours from symptom onset) for any patient with suspected or confirmed influenza who is hospitalized; has severe, complicated, or progressive illness; or is at higher risk for influenza complications.
-
Special considerations for populations at risk for
avian influenza A:
-
Healthcare providers should consider the possibility of avian influenza A
virus infection in a patient with signs and symptoms consistent with acute respiratory tract infection or conjunctivitis and
history of exposure in the last 10 days to animals suspected or confirmed to have avian influenza A. For
additional information on reporting suspect avian influenza, specimen collection, testing & treatment, see
our
September Avian Influenza A Health Advisory.
- Healthcare providers should recommend that patients working with ill animals use
personal protective equipment (PPE) (PDF) and suggest they get a seasonal flu vaccine.
Respiratory Syncytial Virus (RSV)
The following actions are recommended for RSV prevention:
The RSV vaccine is not currently an annual vaccine, meaning adults who already received an RSV vaccine are not recommended to receive additional doses. Additional CDC surveillance and evaluation activities are ongoing to determine whether adults might benefit from receiving additional doses in the future. So far, RSV vaccines appear to provide some protection for at least two RSV seasons. RSV vaccine can be given year-round, but providers are encouraged to maximize the benefit by giving vaccine in late summer or early fall.
Healthcare providers should be aware of chronic medical conditions and
risk factors that may increase the risk of severe RSV illness, and who might be most likely to benefit from these new vaccines.
-
Vaccination for pregnant persons:
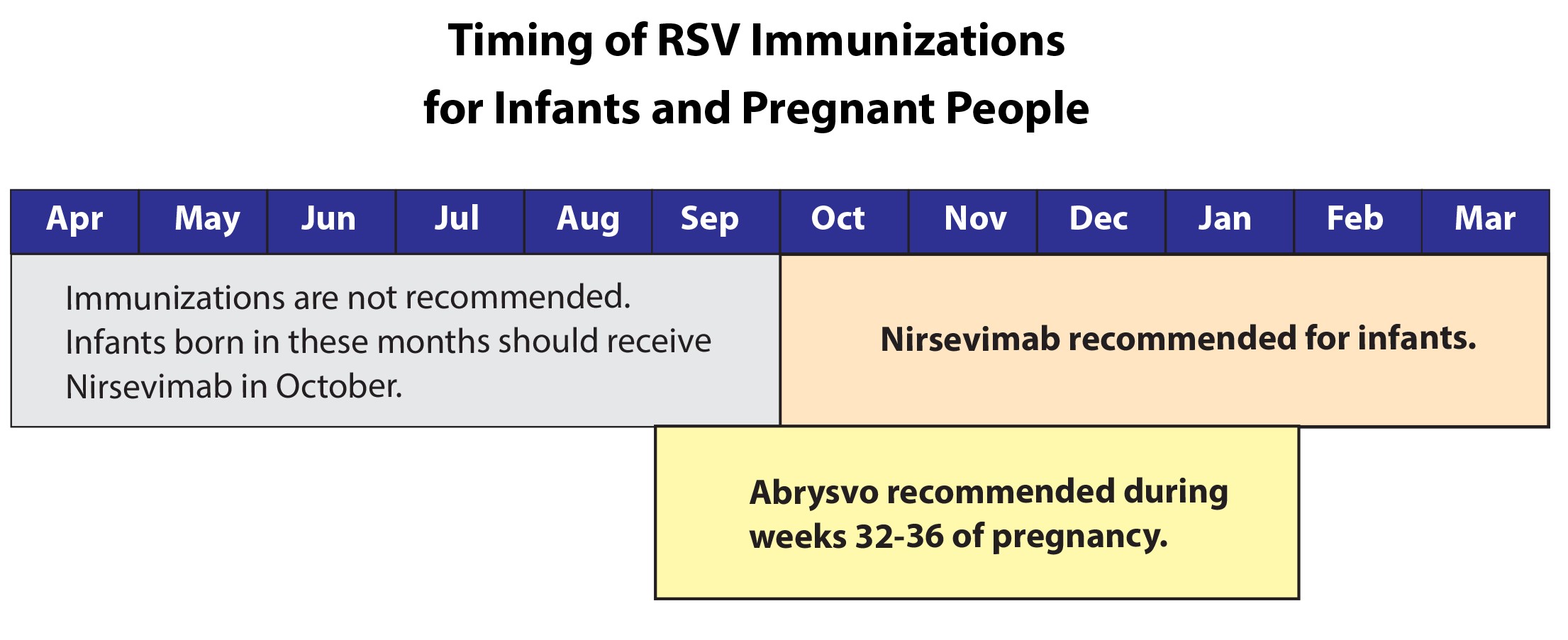
A vaccine for pregnant persons to prevent severe RSV illness in infants is recommended by the Advisory Committee on Immunization Practices (ACIP) and CDC. Pregnant people should receive RSV vaccine during weeks 32 through 36 of pregnancy from September through January so that their babies are protected against severe RSV disease at birth.
People who previously received maternal RSV vaccine are not currently recommended to receive additional vaccine doses during future pregnancies. Those infants should receive nirsevimab.
-
Preventive Monoclonal Antibody Products (Passive Immunization) for Infants and Young Children:
Nirsevimab (Beyfortus), a long-acting monoclonal antibody product, is recommended for prevention of severe RSV lower respiratory tract disease in infants and young children.
Either maternal vaccination or monoclonal antibody is recommended to protect infants against severe RSV disease, but administration of both is not needed for
most infants.
Nirsevimab can provide protection for at least 5 months (the average length of one RSV season), and only one dose is recommended for an RSV season.
Following pre-COVID-19 pandemic patterns, nirsevimab could be administered from October through the end of March. In accordance with
general best practices for immunization, simultaneous administration with age-appropriate vaccines is recommended.
All infants younger than 8 months who are born during – or entering – their first RSV season should receive one dose of nirsevimab. Infants born shortly before or during the RSV season should receive nirsevimab within 1 week of birth. Nirsevimab administration can occur during the birth hospitalization or in the outpatient setting.
Children between the ages of 8 and 19 months who are at increased risk of severe RSV disease are recommended to receive one dose of nirsevimab at the start of their second RSV season.
Palivizumab (Synagis) is another monoclonal antibody product that may be used to protect eligible high-risk infants and children if nirsevimab is not available.
Other vaccines to prevent respiratory disease
Staying up to date with vaccines against pneumococcal disease, pertussis, and other diseases can also reduce the risk of pneumonia and severe outcomes from infection.
Pneumococcal disease is any type of illness caused by Streptococcus pneumoniae bacteria. Some studies have shown an association between increased risk of developing invasive pneumococcal disease and influenza or RSV infection. CDC recommends
pneumococcal vaccination for children under 5 years, adults 65 years or older, and those at increased risk for pneumococcal disease.
Infection Control Measures
During periods of increased transmission of respiratory viruses and in the event of a facility outbreak, healthcare providers facilities should consider implementing source control masking policies as described in CDPH’s
Guidance for Face Coverings as Source Control in Healthcare Settings and in accordance with their local health department recommendations or requirements.
For more information on infection prevention and control of respiratory viruses in skilled nursing facilities, healthcare providers may visit the
CDPH Recommendations for Prevention and Control of COVID-19, Influenza, and Other Respiratory Viral Infections in California Skilled Nursing Facilities (PDF).
Diagnostic Testing to Guide Treatment and Clinical Management
Diagnostic testing is important because it can guide early appropriate antiviral treatment.
Test patients with suspected respiratory virus infections:
-
Especially those with factors placing persons at high risk for severe outcomes from influenza and COVID-19
-
Those with severe or progressive illness
-
Those with potential exposures to animals infected with avian influenza A.
Molecular assays are recommended when testing for RSV, influenza, SARS-CoV-2 in hospitalized patients; testing for other respiratory viruses should be considered since concomitant infections can cause severe illness.