This document provides guidance for health care providers regarding diagnostic tests for SARS-CoV-2, the virus causing COVID-19 disease. While serologic tests are becoming more widely available, no serologic tests are currently approved by the FDA for diagnosing COVID-19. Reverse transcriptase-polymerase chain reaction (RT-PCR) tests remain the preferred tests for diagnosing COVID-19 in individual patients.
Serologic tests should generally not be used to diagnose acute cases of COVID-19 or to infer immunity, because:
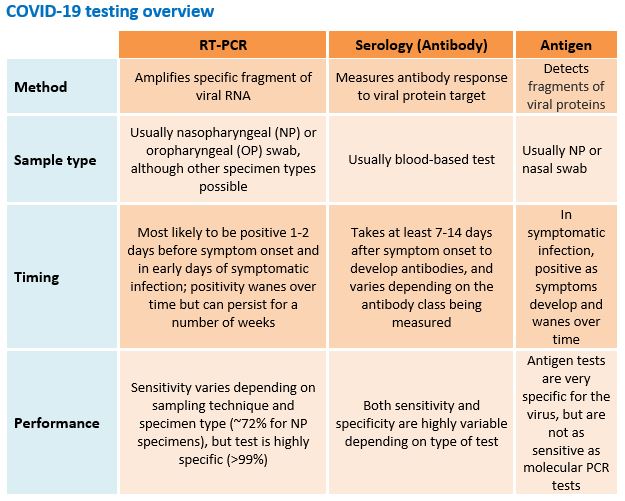
- There is a lag in production of antibodies. It can take 7-14 days after symptom onset for antibodies against SARS-CoV-2 to be detectable by serologic assays. This delay means that these tests are not useful in making the diagnosis of acute disease.
- A positive IgM test does not mean current infection. While presence of IgM is often considered a sign of current infection, the lag between symptom onset and antibody production against SARS-CoV2 means that detection of IgM may not indicate acute infection. Studies suggest that detection of IgM can lag symptom onset by 12 days or more, and fewer than 40% of patients will have detectable antibodies in the first week of infection.
- A positive serologic test may not mean a patient is immune. A number of different SARS CoV-2 antibodies may be produced during an immune response. It is not clear which antibodies confer immunity or how long persons who develop detectable antibodies are protected against later reinfection with SARS-CoV-2. Until more information is available, patients with a positive serologic assay should not be assumed to be immune to SARS CoV-2.
There is a high degree of variability in sensitivity and specificity between different serologic assays. Serologic tests are generally less specific than RT-PCR tests and have a greater potential to cross-react with Coronaviruses other than SARS-CoV-2. Remember that the positive predictive value of a test depends on not only the sensitivity and specificity of a test, but also on prevalence of disease. In a population with a 5% prevalence of SARS-CoV-2 infection, a serologic test with 95% sensitivity and 95% specificity will have 50% positive predictive value.
What types of serology tests are available?
- There are many different serology tests for COVID-19 with variable performance characteristics.
- FDA maintains a list of in vitro diagnostic tests for COVID-19 granted Emergency Use Authorization (EUA).
- The California Department of Public Health does not recommend use of serology tests that are not authorized under EUA.
- Serologic tests vary in the following ways:
- Rapid tests vs. ELISA-based tests: Rapid tests are typically point-of-care tests similar to pregnancy tests, with qualitative (positive or negative) results. Enzyme-linked immunosorbent assay (ELISA) tests can be qualitative or quantitative and are usually lab-based tests. Note that rapid tests are validated with serum (from a venous blood draw), but are often less sensitive and specific when performed on capillary blood (i.e., from a finger-prick).
- Types of antibody: Serologic tests may measure levels of IgM, IgG, IgA, or a combination of these. Antibody dynamics and correlation to protective immunity is not fully understood.
- Target viral antigens: Tests vary in the viral antigen(s) they target, for example the nucleoprotein (N protein) or spike protein (S protein). The clinical significance of each target, and correlation to protective immunity, is not fully understood.
When might serologic testing be appropriate?
- Serologic tests are most appropriate as a surveillance tool (i.e., providing population-level estimates of exposure to SARS-CoV-2), rather than as a diagnostic tool for individual patients.
- Serology tests may be used in individual patients with negative RT-PCR testing but a high clinical suspicion for COVID-19 disease. An example might include:
- A hospitalized patient, 12 days after symptom onset of fever and non-productive cough, found to have ground glass opacities on chest imaging and progressive respiratory failure, whose initial SARS-CoV-2 RT-PCR testing from pooled OP + NP swab was negative. Respiratory viral panel testing was also negative, and no other cause of respiratory failure has been identified. Patient is currently on hi-flow oxygen therapy, and a lower respiratory tract specimen for repeat SARS-CoV-2 RT-PCR test is difficult to obtain.
- In the future, serology tests may be used to document seropositivity for plasma donation or vaccine studies, although as above, the specific antibody responses that confer protective immunity are not fully understood.
- A positive COVID-19 serologic test may be consistent with any of the following:
- Recent SARS-CoV-2 infection, whether symptomatic (>1 week after onset of symptoms) or asymptomatic
- Past infection, whether symptomatic or asymptomatic
- False positive results and no COVID-19 infection
- A negative COVID-19 serologic test may be consistent with any of the following:
- No recent or prior SARS-CoV-2 infection
- Early SARS-CoV-2 infection, after exposure but prior to the development of antibodies
- False negative results after true COVID-19 infection
What about antigen testing?- The California Department of Public Health does not recommend use of antigen tests that are not authorized under EUA.
- These rapid tests identify viral protein fragments in NP and nasal swabs from suspect cases of COVID-19. They are authorized for use in high and moderate complexity laboratories certified by Clinical Laboratory Improvement Amendments (CLIA), as well as for point-of-care testing by facilities operating under a CLIA Certificate of Waiver.
- An antigen test can provide results in minutes; however, it is not as sensitive as PCR tests. This means that positive results tend to be highly accurate, but there is a higher chance of false negatives. Thus, negative results do not rule out infection and may need to be confirmed by PCR.
Other considerations- Are serology results reported to public health departments? Serology results are currently reported to local and state health departments via electronic lab reporting (ELR), but positive results are not counted as confirmed COVID-19 cases. Local health departments may use serology results as needed for surveillance and tracking probable cases, but are not required to take any actions based on serology results.
- FDA maintains a list of in vitro diagnostic tests for COVID-19 granted Emergency Use Authorization (EUA).
- Fraudulent test kits. The FDA has reported on the sale of unauthorized fraudulent test kits for COVID-19. At this time, there are no kits approved for at-home testing of COVID-19. Please counsel patients to avoid home testing.
Where can I find more information?