Compliance Information for Cosmetics Companies and Frequently Asked Questions
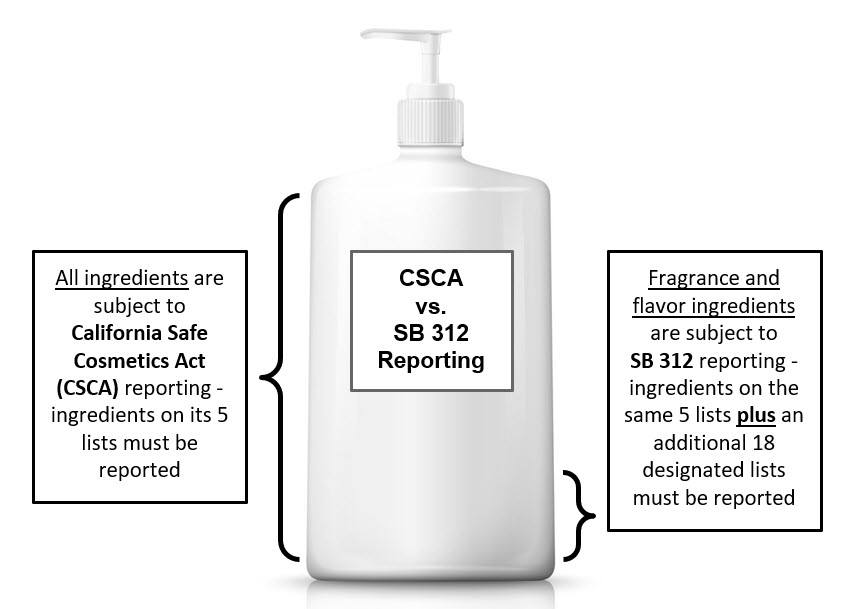
California Safe Cosmetics Act (CSCA)
Senate Bill 484 (SB 484), now known as the California Safe Cosmetics Act of 2005 (CSCA), went into effect January 1, 2007 and requires cosmetics manufacturers to disclose to the California Department of Public Health (CDPH) all products sold in California containing ingredients listed by the five authoritative scientific bodies cited in the
California Health and Safety Code Section 111791.5.
Cosmetic Fragrance and Flavor Ingredient Right to Know Act of 2020 (CFFIRKA)
Senate Bill 312 (SB 312), now known as the Cosmetic Fragrance and Flavor Ingredient Right to Know Act of 2020 (CFFIRKA), went into effect on January 1, 2022. CFFIRKA can be found in Health and Safety Code Section 111792.6. As of that date, cosmetic products sold in the state must be reported to CPDH if they contain reportable fragrance and flavor ingredients. CSCP consolidates all reportable ingredients under CSCA and CFFIRKA in its
CSCP Reportable Ingredients List [Excel], which is updated twice annually. When the Reportable Ingredients List is updated, companies have 6 months to comply.
Fragrance Allergens
The subset of CFFIRKA reportable ingredients called “fragrance allergens” have a distinct reporting requirement. Fragrance allergens, unlike other fragrance ingredients, must be reported regardless of their intended purpose in the product, i.e. they must be reported even if they are not used to impart scent or counteract odor. Additionally, fragrance allergens must be reported when present in a rinse-off cosmetic product at a concentration at or above 0.01 percent (100 parts per million) or in a leave-on cosmetic product at a concentration at or above 0.001 percent (10 parts per million). Fragrance allergen ingredients are clearly distinguished in the CSCP Reportable Ingredients List. All other ingredients appearing on the Reportable Ingredients List must be reported regardless of concentration in the product.
Please note, as of March 27, 2024, the Reportable Ingredient List's Summary of Updates tab has an asterisk added next to each of the European Union's (EU) newly listed fragrance allergens. The following information applies to the ingredients with the asterisk: A manufacturer of a cosmetic product sold in the state shall report a list of each fragrance allergen included in Annex III of the EU Cosmetics Regulation No. 1223/2009, as required to be reported pursuant to the EU Detergents Regulation No. 21 648/2004. Manufacturers must report listed allergens to the California Department of Public Health no later than the timelines required under the EU law, i.e., 2026 or 2028; however, manufacturers may report at any time prior to the deadlines.
The California Safe Cosmetics Reporting Portal
The California Safe Cosmetics Reporting Portal, previously used only for CSCA reporting, was updated to accommodate CFFIRKA reporting as well. If you cannot find the information you are seeking here, please check the Cosmetic Companies page for instructions on using the system or email SafeCosmetics@cdph.ca.gov.
 Hidden Text
Hidden Text
Frequently Asked Questions about CSCA and CFFIRKA
Frequently Asked Questions about the Online Reporting System
Your company is subject to the mandatory reporting requirements of CSCA and CFFIRKA if its products are sold in California and contain ingredients appearing on one or more of the authoritative lists of hazardous substances in the laws. Please see our Who Needs to Submit page for more details.
Which products and which ingredients must be reported?
Any cosmetic product sold in California, which contains an ingredient known or suspected of causing harm must be reported. More information on this topic can be found on our Cosmetic Companies web page. CSCP compiles and regularly updates a list of all reportable ingredients under CSCA and CFFIRKA:
The company whose name appears on the product label pursuant to the requirements of Section 701.12 of Title 21 of the Code of Federal Regulations is responsible for reporting products to CSCP, and for obtaining ingredient information from manufacturers and suppliers necessary to make the report. Consultants or other third parties may report on behalf of the responsible company, but CSCP will first verify that consultants are legitimately affiliated with the company.
The company responsible for reporting is required to obtain the information necessary to report from all suppliers.
All cosmetic products with reportable ingredients sold in California after Jan. 1, 2022, regardless of date of manufacture, must be reported under this mandate.
Companies were expected to start the process of reporting by Jan. 1, 2022. The
California Safe Cosmetics Reporting Portal is now ready for CFFIRKA reporting.
No. There is no requirement in these laws to make changes to product labels.
No. The laws do not require companies to report any of the following: ingredients not included in the designated lists, the weight or amount of an ingredient, or how a product is formulated (i.e. the recipe).
All UPCs associated with a product containing reportable ingredients must be reported, regardless of how it is packaged. If a product containing a reportable ingredient is part of a larger kit or collection, the company is required to report the kit’s UPC and the UPC of any product that is also sold separately and contains the reportable ingredient.
Are trade secrets (TS) exempt from reporting? What kind of supporting documentation will I need to submit to substantiate my trade secret claim?
TS information is not exempt from reporting to CDPH. However, CDPH cannot make any confidential and/or TS information available to the public unless the outcome of an official investigation deems the information is not confidential. Companies declaring TS for any ingredient must submit supporting written documentation to justify this designation.
For purposes of CSCA, TS designation is determined “pursuant to the procedure established in Part 20 and Section 720.8 of Part 720 of Title 21 of the Code of Federal Regulations” (California Health and Safety Code § 111791). The CSCP requires documentation from the US Food and Drug Administration (US FDA) granting TS status, indicating that the information so designated is indeed TS under federal law.
For an ingredient to qualify as a TS, you must (1) provide written documentation from US FDA granting the ingredient TS status and (2) provide proof that the ingredient(s) meet the Uniformed Trade Secrets Act’s (California Civil Code § 3426.1) definition of “trade secret.” Documentation must be submitted for each ingredient you are seeking to designate as TS.
The ingredient(s) must also meet the definition of “trade secret” under California law. Under the California Uniformed Trade Secrets Act, "trade secret" is defined as information, including a formula, that (1) derives independent economic value from not being generally known to the public or to other persons who can obtain economic value from its disclosure or use; and (2) is the subject of efforts that are reasonable under the circumstances to maintain its secrecy. Note that ingredients listed on the label of products sold to the public are “known to the public” and therefore would not meet the definition of a TS. Additionally, pursuant to California Health and Safety Code § 111792.6, a fragrance ingredient or flavor ingredient that is included in a designated list, or a fragrance allergen that requires disclosure pursuant to subparagraph (B) of paragraph (1), does not constitute a trade secret.
Cosmetics companies should report electronically using the California Safe Cosmetics Reporting Portal. Instructions detailing the submission process are available on our Cosmetics Companies web page.
How do I update my company’s information in the Reporting Portal?
You can edit company contact information or user profile online.
To edit your company’s information in the Reporting Portal:
-
Log in to your account.
-
Click the “My Companies” tab in the left navigation pane, then click the “Edit” button.
-
Make the desired changes.
-
Hit “Submit” to override your original entry.
Yes, anyone with an account linked to a company can edit products they submitted or product submitted by others for that company online in the Reporting Portal. Most elements of a product submission can be edited, with the exception of the Company, Brand, Product and Variant name combination, Ingredient Name, and CAS#.
To edit a product entry:
-
Log in to your account.
-
Click the “My Products” link.
-
Find the entry you want to edit from your submitted product list, and click on the “Edit” button to access your submission.
-
Click through the submission, making the desired changes to each screen. NOTE: Hitting the “Continue” button at the bottom of each page saves the data on that page. Any changes made to a page will overwrite existing data when you hit “Continue.”
Yes, you can remove an ingredient from a product IF that product has been reformulated and no longer contains that ingredient. Follow the instructions provided for editing a submission (see above). Use the “Remove” button instead of the “Edit” button and follow the prompt to enter the date the ingredient was removed from the product and hit “Remove Ingredient” to make this change.
I received an error message while submitting to the database. What happened and what should I do?
The most common reason for receiving an error message while submitting data is not completing all required fields on the page. If you get an error message, review the questions on the page and provide any missing information for all data fields on that page. If this does not resolve your problem, contact us at
SafeCosmetics@cdph.ca.gov. Include both the error message you received and a detailed description of what you were doing when you received the error in the body of your email.
* If you are having difficulty accessing this document, please contact safecosmetics@cdph.ca.gov to request this information in an alternate format.
** Si usted tiene dificultades de acceso con este documento, favor de ponerse en contacto con safecosmetics@cdph.ca.gov para pedir esta información en un formato diferente